Abstract
Recent studies have suggested that epigenetic inactivation of tumour-related genes by promoter methylation participates in the development of gastric cancer. We newly identified the frequently aberrant promoter methylation of alpha-1B-adrenergic receptor (ADRA1B) in colorectal cancer by methylation-sensitive representational difference analysis (MS-RDA) and examined the methylation status of the ADRA1B promoter in 34 paired samples of colorectal cancer and surrounding epithelial tissue, and 34 paired samples of gastric cancer and surrounding epithelial tissue. In colorectal cancers, only four of 34 (11.8%) tumours showed ADRA1B promoter methylation. In contrast, ADRA1B promoter methylation was detected in 24 of 34 (70.6%) gastric cancers and in 14 of 34 (41.2%) surrounding epithelial tissues. The frequency of ADRA1B promoter methylation was higher in gastric epithelial tissues with intestinal metaplasia (41.6%) than in those without intestinal metaplasia (25.0%). Reverse transcription–PCR detected reduced ADRA1B expression in 12 of 18 (66.7%) gastric cancers, and its promoter methylation was detected in 11 of these 12 (91.7%) gastric cancers with reduced ADRA1B expression. Thus, ADRA1B promoter is frequently methylated in gastric cancer. Our results suggest that the ADRA1B gene is an important tumour-related gene frequently involved in the development and progression of gastric cancer.
Keywords: ADRA1B , gastric cancer, intestinal metaplasia, DNA methylation
Aberrant DNA methylation is a feature of human cancers, characterised by generalised hypomethylation and regional hypermethylation (Gama-Sosa et al, 1983; Feinberg et al, 1988). When regional hypermethylation occurs in CpG sites within the promoter region of tumour-suppressor genes or tumour-related genes that normally are unmethylated, gene transcription is inhibited, similar to the effects of mutations and deletions (Herman et al, 1994; Merlo et al, 1995; Baylin and Herman, 2000; Esteller et al, 2001). There is a growing list of tumour-suppressor genes or tumour-related genes associated with CpG island methylation in cancers (Leung et al, 1999; Kang et al, 2000; Shim et al, 2000; Kaneda et al, 2002a, 2002b; Li et al, 2002; Byun et al, 2003; Chan et al, 2003; Tokumaru et al, 2003; Choi et al, 2004; Miotto et al, 2004).
Gastric cancer is the leading cause of cancer-related mortality in Japan and many other countries (Fuchs and Mayer, 1995). Although the molecular genetics of gastric cancer remain unclear, accumulating evidence suggests that many tumour-suppressor genes and tumour-related genes are inactivated by promoter methylation (Leung et al, 1999; Kang et al, 2000; Shim et al, 2000; Kaneda et al, 2002a, 2002b; Li et al, 2002; Byun et al, 2003; Chan et al, 2003; Tokumaru et al, 2003; Choi et al, 2004; Miotto et al, 2004). Among various genes, methylation in the CpG island of the hMLH1 gene, which encodes for the DNA mismatch repair protein MLH1, has been linked to a substantial proportion of sporadic gastric cancers with microsatellite instability (Fleisher et al, 1999; Leung et al, 1999). Some gastric cancers are characterised by a high degree of concordant methylation of CpG islands, including p16, E-cadherin, and hMLH1; such tumours are classified as high CpG island methylator phenotype (CIMP) (Toyota et al, 1999). Epigenetic inactivation of tumour-related genes by promoter methylation thus seems to have an important role in the development of gastric cancer.
To enable a genome-wide search for differences in CpG methylation between cancer and normal tissue, methylation-sensitive representational difference analysis (MS-RDA) was developed by Ushijima et al (1997). This method demonstrated reduced expression of the INSIG1 gene and possible silencing of the p41Arc gene due to promoter methylation in gastric cancer (Kaneda et al, 2002a). In addition, several genes were also shown to be inactivated by aberrant promoter methylation in human cancers (Takai et al, 2001; Kaneda et al, 2002b; Asada et al, 2003). In this study, we used the MS-RDA technique to analyse two human colorectal cancers and newly identified alpha-1B-adrenergic receptor (ADRA1B). In colorectal cancers, only a subset of tumours showed aberrant ADRA1B promoter methylation. In contrast, ADRA1B promoter methylation was found much more frequently not only in gastric cancers but also in their surrounding epithelial tissues, and the majority of gastric cancers with ADRA1B promoter methylation had reduced ADRA1B expression. Our results suggest that aberrant ADRA1B promoter methylation with a consequent reduction in ADRA1B expression may be involved in gastric carcinogenesis.
MATERIALS AND METHODS
Clinical materials
Thirty-four paired samples of colorectal cancer and surrounding epithelial tissue, and 34 paired samples of gastric cancer and surrounding epithelial tissue were obtained at the time of surgery with informed consent. In addition, three samples of gastric epithelial tissue free of gastric cancer were obtained from the patients who underwent pancreaticoduodenectomy for the treatment of pancreatic cancer. Samples were immediately frozen in liquid nitrogen and stored at −80°C until DNA and RNA extraction. Among the 34 samples of surrounding gastric epithelial tissue, intestinal metaplasia (IM) was found in 26 (76.5%) on histopathological examination.
Mmethylation-sensitive representational difference analysis, sequencing, and database search
Methylation-sensitive representational difference analysis was performed as described by Ushijima et al (1997), using DNA obtained from two paired samples of colorectal cancer and surrounding epithelial tissue. Briefly, genomic DNAs of cancer and surrounding epithelial tissue were digested by HpaII (New England Biolabs, Beverly, MA, USA), and the Rhpa adaptor was ligated to the digest. HpaII-amplicon was prepared by PCR. The Rhpa adaptor of the HpaII-amplicon from the corresponding sample of normal tissue was removed by MspI digestion, gel-purified (Gel Extraction Kit; Qiagen, Hilden, Germany), and switched to JHpa adaptor. The HpaII-amplicon from the surrounding epithelial tissue was mixed with an excess amount of that from cancer tissue to perform competitive hybridisation, followed by PCR with JHpa primer. After two cycles of competitive hybridisation, the products were cloned into pGEM-T Easy vector (Promega, Madison, WI, USA). Then, plasmid DNA was cycle sequenced with the SP6 and T7 primers, using a CEQ Dye Terminator Cycle Sequencing Quick Start Kit (Beckman Coulter, Inc., Fullerton, CA, USA), and a CEQ2000XL DNA analyser (Beckman Coulter, Inc.). Homology searches were performed with BLAST program at the GenBank web site.
Methylation-specific PCR for ADRA1B promoter in colorectal and gastric cancers and surrounding epithelial tissues
We performed methylation-specific PCR (MSP) to determine the methylation status of ADRA1B promoter in 34 paired samples of colorectal cancer and surrounding epithelial tissue and 34 paired samples of gastric cancer and surrounding epithelial tissue, using bisulphite-modified genomic DNA as described by Herman et al (1996). In brief, 1 μg of DNA was denatured by NaOH and modified by sodium bisulphite. The DNA sample was then purified with Wizard DNA purification resin (Promega Corp.), treated again with NaOH, ethanol precipitated, and resuspended in H2O. We used four primer sets (Region 1, nucleotides −590 to −506; Region 2, nucleotides −517 to −274; Region 3, nucleotides −323 to −213; and Region 4, nucleotides −225 to −61) to comprehensively investigate the methylation status of ADRA1B promoter (nucleotides −754 to +173) (Ramarao et al, 1992). Because Region 2 includes a very wide area, which contains many CpGs, we set MSP for Region 3, which extensively overlaps with the 3′ end of the Region 2. The transcription start site was defined as +1, and the primer sets and PCR conditions are described in Table 1. Human genomic DNA treated in vitro with SssI methylase (New England Biolabs, Inc, Beverly, MA, USA) was used as positive control. The PCR products were analysed on 2% agarose gels with ethidium bromide and visualised under UV illumination. The presence of a visible PCR product in sets for methylated specific DNA was judged to be methylation-positive.
Table 1. Primer sets and PCR conditions of methylation-specific PCR for ADRA1B promoter.
| Sense primer | Antisense primer | Amplicon size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| Region 1 | ||||
| (M) GGGTGATTCGCGATTTTTAC | CTCCCAAATCACCTCTACGA | 83 | 56 | 40 |
| (U) GTGGGTGATTTGTGATTTTTATGT | CTCCCAAAATCACCTCTACAAA | 85 | 56 | 40 |
| Region 2 | ||||
| (M) CGTTTAAGGTTCGTTTTCGC | AAAAAAATCTACTTCAATAAACCGCT | 243 | 56 | 40 |
| (U) TTATGTTTAAGGTTTGTTTTTGTGG | AAAAAATCTACTTCAATAAACCACT | 245 | 54 | 40 |
| Region 3 | ||||
| (M) TGGATTCGTATTGTTTTTTAGTGTC | AAAAAATCTACTTCAATAAACCGCT | 110 | 58 | 35 |
| (U) TTGGATTTGTATTGTTTTTTAGTGTGTTG | AAAAAATCTACTTCAATAAACCACT | 111 | 58 | 35 |
| Region 4 | ||||
| (M) AAGTAGATTTTTTTCGGCGTTC | AACTCCAAATTTAATAATCCACGTC | 165 | 60 | 35 |
| (U) AGTAGATTTTTTTTGGTGTTTGT | AACTCCAAATTTAATAATCCACATC | 165 | 60 | 35 |
(M)=methylated DNA specific; (U)=unmethylated DNA specific; ADRIB=alpha-1B-adrenergic receptor; PCR=polymerase chain reaction.
Bisulphite sequencing of ADRA1B promoter in gastric cancers and surrounding epithelial tissues
We performed bisulphite sequencing of ADRA1B promoter in 10 randomly selected paired samples of gastric cancer and surrounding epithelial tissue. Bisulphite-modified DNA was used for PCR with primers common for methylated and unmethylated DNA sequences, which amplified a product containing 68 CpG sites (nucleotides −672 to −59) in ADRA1B promoter. The primer sets and PCR conditions are described in Table 2. The PCR products were gel-purified (Gel Extraction Kit; Qiagen, Hilden, Germany) and were cloned into pGEM-T Easy vector (Promega). Eight recombinants were cycle sequenced with the SP6 and T7 primers, using a CEQ Dye Terminator Cycle Sequencing Quick Start Kit and a CEQ2000XL DNA analyser (both from Beckman Coulter, Inc.). The methylation status of each CpG site was determined by sequencing, as unmethylated cytosines are converted into thymines by bisulphite treatment, whereas methylated cytosines remain unaltered.
Table 2. Primer set and PCR conditions of bisulphite sequencing for ADRA1B promoter.
| Sense primer | Antisense primer | Amplicon size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| TATTAAAGGTAAGTAGTTTTTAATTTATTT | ACAACTCCAAATTTAATAATCCAC | 614 | 58 | 40 |
ADRA1B=alpha-1B-adrenergic receptor; PCR=polymerase chain reaction.
Semiquantitative reverse transcription(RT)–PCR
Total RNA was prepared from 18 paired samples of gastric cancer and surrounding epithelial tissue for which the methylation status of ADRA1B promoter had been assessed by MSP. The total RNA was immediately treated with DNase I (Life Technologies, Rockville, MD, USA) and reverse-transcribed using a Superscript III reverse transcriptase kit (Life Technologies) to prepare first-strand cDNA. A β-actin fragment was amplified as an internal control. The primer set and PCR conditions are described in Table 3.
Table 3. Primer set and PCR conditions of RT–PCR for ADRA1B expression.
| Sense primer | Antisense primer | Amplicon size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| GCTAAGACGTTGGGCATTGT | GTTGAAGTAGCCCAGCCAGA | 144 | 60 | 35 |
ADRA1B=alpha-1B-adrenergic receptor; PCR=polymerase chain reaction; RT=reverse transcription.
5q loss of heterozygosity analysis
5q loss of heterozygosity (LOH) analysis was carried out using a single-nucleotide polymorphism (SNP) in the ADRA1B gene (5q23–q32), three SNPs in the adenomatous polyposis coli gene (5q21–q22), and an SNP in the interferon regulator factor-1 gene (5q31.1) for the 18 paired samples of gastric cancer and surrounding epithelial tissue examined by RT–PCR. Detailed information about these five SNPs is available from JSNP (http://snp.ims.u-tokyo.ac.jp). Sequence change in SNP from the PCR product of surrounding epithelial tissue to that from the cancer tissue was judged as 5q LOH positive. The primer sets and PCR conditions are described Table 4; the primers for PCR were used as sequence primers.
Table 4. Primer sets and PCR conditions for the region including SNP in ADRA1B, APC, and IRF-1.
| Sense primer | Antisense primer | Amplicon size (bp) | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| ADRA1B (JSNP ID; IMS-JST087433: A4154019G) | ||||
| CTGGTCACGCGGAGGAAG | GGTTCTTGGTGGTTCTCTTTGG | 244 | 60 | 35 |
| APC (JSNP ID; IMS-JST061883: C113263G) | ||||
| TGCTTGAAAATTCCAGTGTCA | GGACATTTTTGACCGCAGTT | 382 bp | 62 | 35 |
| APC (JSNP ID; IMS-JST041076: A14531742C, and JSNP ID; IMS-JSTO41075: G14531786A) | ||||
| GCCAGGATATGGAAAAACGA | TTCCAAGGCAGAACAGAACA | 255 bp | 62 | 35 |
| IRF-1 (JSNP ID; IMS-JST005685: T34238200C) | ||||
| ATCAGCAGCCAGAGGGTAGA | CTGGCAAAAGCATCTGTGAA | 231 bp | 62 | 35 |
ADRA1B=alpha-1B-adrenergic receptor; PCR=polymerase chain reaction.
Statistical analysis
The Fishers' exact test and Student's t-test were used to examine associations between two categorical variables. The level of statistical significance was set at P<0.05.
RESULTS
Isolation of DNA fragments aberrantly methylated in colorectal cancer
DNAs from two paired samples of colorectal cancer and surrounding epithelial tissue were used as tester and driver for MS-RDA. We obtained 33 DNA fragments by MS-RDA, and the genomic origins of the 33 DNA fragments were analyssed by sequencing and a GenBank database search. One DNA fragment matched the promoter of the ADRA1B gene, which is located in 5q23–q32.
ADRA1B promoter methylation in colorectal and gastric cancers and surrounding epithelial tissues
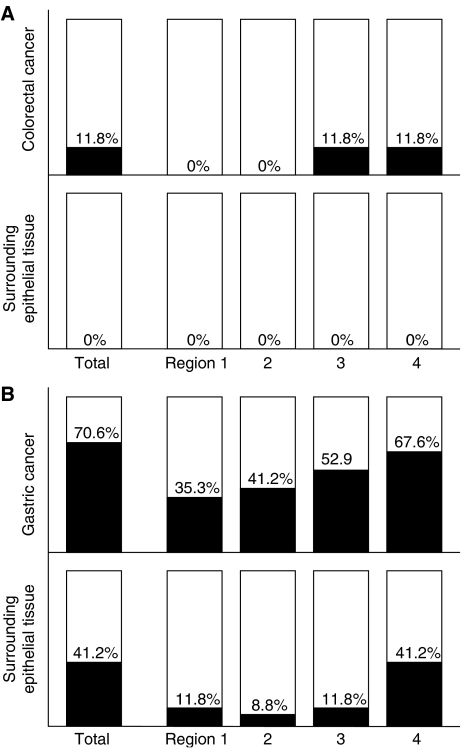
As shown in Figure 1A, ADRA1B promoter methylation was detected by MSP in four of 34 (11.8%) colorectal cancers. In all four of these colorectal cancers, methylation was limited to Region 3 and Region 4 in ADRA1B promoter. In contrast, no ADRA1B promoter methylation was detected in the 34 corresponding samples of normal colorectal epithelial tissue. Thus, ADRA1B promoter methylation was a cancer-specific event in the colorectal cancers and surrounding epithelial tissues.
Figure 1.
Alpha-1B-adrenergic receptor promoter methylation was detected in four of 34 (11.8%) colorectal cancers and was not detected in surrounding colorectal epithelial tissues. Alpha-1B-adrenergic receptor promoter methylation was thus considered a cancer-specific event in colorectal cancers and surrounding epithelial tissues. In contrast, ADRA1B promoter methylation was detected in 24 of 34 (70.6%) gastric cancers and in 14 of 34 (41.2%) surrounding epithelial tissues. In gastric cancers, the frequency of ADRA1B promoter methylation increased progressively from Region 1 to Region 4. In surrounding epithelial tissues, the frequency of ADRA1B promoter methylation was also highest in Region 4.
As shown in Figure 1B, ADRA1B promoter methylation was detected in 24 of 34 (70.6%) gastric cancers and in 14 of 34 (41.2%) surrounding epithelial tissues. Alpha-1B-adrenergic receptor promoter methylation in gastric cancers and in surround epithelial tissues showed no significant correlation with clinicopathological characteristics such as age and gender of the patients or the location, stage, and differentiation of their tumours. Alpha-1B-adrenergic receptor promoter methylation was not detected in the three samples of gastric epithelial tissue obtained from patients with pancreatic cancer unassociated with gastric cancer. Among the 34 gastric cancers, ADRA1B promoter methylation was detected in Region 1 in 12 cancers (35.3%), Region 2 in 14 (41.2%), Region 3 in 18 (52.9%), and Region 4 in 23 (67.6%). Therefore, in gastric cancers, the frequencies of ADRA1B promoter methylation increased progressively from Region 1 to Region 4. Among the 34 surrounding epithelial tissues, ADRA1B promoter methylation was detected in Region 1 in four samples (11.8%), Region 2 in three (8.8%), Region 3 in four (11.8%), and Region 4 in 14 (41.2%). For each region, the frequency of ADRA1B promoter methylation was lower in the surrounding epithelial tissues than in the cancers; however, similar to the cancers, the frequency of ADRA1B promoter methylation was highest in Region 4 in the surrounding epithelial tissues.
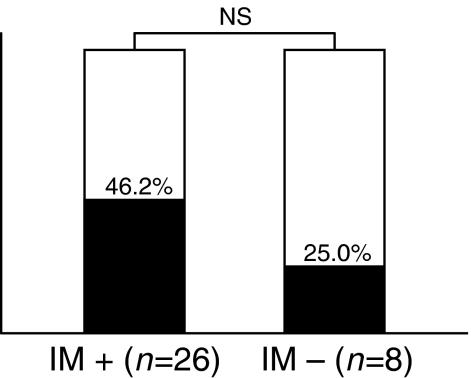
When we analysed the correlations between ADRA1B promoter methylation and the presence of IM in surrounding epithelial tissues, ADRA1B promoter methylation was detected in 12 of 26 (46.2%) gastric epithelial tissues with IM and in two of eight (25.0%) gastric epithelial tissues without IM (Figure 2). The frequency of ADRA1B promoter methylation was slightly but not significantly higher in gastric epithelial tissues with IM than in gastric epithelial tissues without IM.
Figure 2.
The frequency of ADRA1B promoter methylation in gastric epithelial tissues with IM (41.6%) was slightly but not significantly higher than that in gastric epithelial tissues without IM (25.0%).
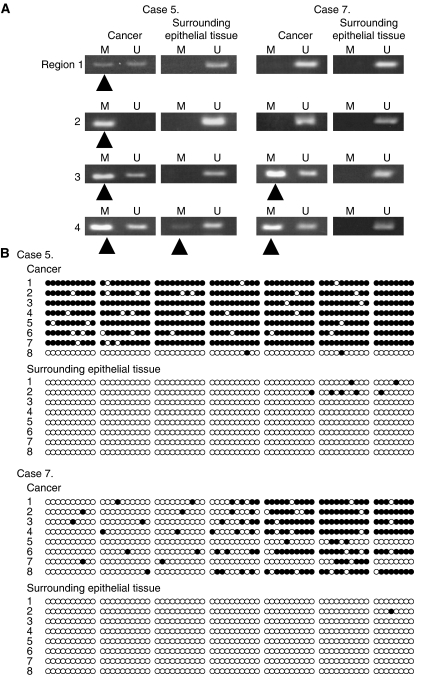
In 10 analysed gastric cancers, the results of bisulphite sequencing of ADRA1B promoter methylation were concordant with those of MSP regarding the extent of the methylation area (Figure 3A and B). Four of 10 samples judged to be methylation-negative on MSP showed sequence changes of nearly all cytosines to thymines in eight recombinants in the ADRA1B promoter on bisulphite sequencing. Six of 10 samples judged to be methylation-positive on MSP exhibited methylation in nearly all eight recombinants in the same region. Seven of 10 analysed samples of surrounding epithelial tissue judged to be methylation-negative on MSP showed sequence changes of nearly all cytosines to thymines in eight recombinants in the ADRA1B promoter on bisulphite sequencing, similar to Case 7. In three of 10 samples of surrounding epithelial tissues judged to be methylation-positive in Region 4 on MSP, bisulphite sequencing showed sequence changes of nearly all cytosines to thymines in eight recombinants in the ADRA1B promoter, similar to Case 5. In most gastric cancers positive for ADRA1B promoter methylation, the band of PCR products obtained with sets for methylated specific DNA was more intense than that obtained with sets for unmethylated specific DNA, similar to Case 5 and Case 7 (Figure 3A). In some of the corresponding epithelial tissues positive for ADRA1B promoter methylation, the band of PCR products obtained with sets for methylated specific DNA was less intense than that obtained with sets for unmethylated specific DNA, similar to Case 5 (Figure 3A). Therefore, even in samples judged to be methylation-positive, the quantity of methylated DNA was less than that of unmethylated DNA in the surrounding epithelial tissues, and this difference may account for the inconsistency between the results of MSP and bisulphite sequencing for the surrounding epithelial tissues.
Figure 3.
Methylation status of ADRA1B promoter was analysed by methylation-specific PCR (MSP) and bisulphite sequencing of 68 CpG sites in eight clones obtained by PCR of bisulphite-modified DNA. (A) Representative results of MSP for two paired samples of gastric cancer and surrounding epithelial tissue. The presence of a visible PCR product in the lanes marked M indicates the presence of methylated alleles (black arrow). (B) Schemas of the results of bisulphite sequencing of the same samples as those analysed by MSP. Unmethylated and methylated cytosines are shown by open and closed circles, respectively. In Case 5, analysis of DNA from cancer tissue showed ADRA1B promoter methylation in Regions 1, 2, 3, and 4 on MSP and widespread methylation in seven of eight clones on bisulphite sequencing. In Case 7, analysis of DNA from cancer tissue showed ADRA1B promoter methylation in Regions 3 and 4 on MSP; methylation in these regions was also revealed by bisulphite-sequencing in six of eight clones. These two cases of cancer also had reduced ADRA1B expression. In Case 7, analysis of DNA from the surrounding epithelial tissues showed no ADRA1B promoter methylation in any region on MSP; similar results were obtained on bisulphite sequencing. In Case 5, analysis of DNA from the surrounding epithelial tissues showed methylation in Region 4 on MSP, but no methylation in any clone on bisulphite sequencing. In these two cases, the surrounding epithelial tissues showed no reduced ADRA1B expression. Although DNA was judged to be methylation-positive in the surrounding epithelial tissues of Case 5, the amount of methylated DNA was probably much less than that of unmethylated DNA, resulting in no apparent methylation on bisulphite sequencing.
Correlation among reduced ADRA1B expression, ADRA1B promoter hypermethylation, and 5q LOH in gastric cancers and surrounding epithelial tissues
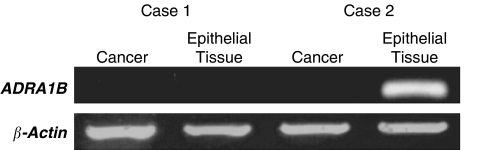
Semiquantitative RT–PCR detected reduced ADRA1B expression in 12 of 18 (66.6%) gastric cancers and three of 18 (16.4%) surrounding tissues (Figure 4). Table 5 shows correlations among ADRA1B promoter methylation status according to region, 5q LOH, and ADRA1B expression in 18 paired samples of gastric cancer and surrounding epithelial tissue. All three cases with reduced ADRA1B expression in surrounding epithelial tissue also had reduced ADRA1B expression in their corresponding cancers. Eleven of 12 (91.7%) gastric cancers and three of three (100.0%) corresponding tissues with reduced ADRA1B expression also had promoter methylation in Region 2 and/or Region 3, and three of 11 (27.3%) gastric cancers with reduced ADRA1B expression also had 5q LOH. On the other hand, three gastric cancers and six surrounding epithelial tissues with ADRA1B promoter methylation did not have reduced ADRA1B expression. In most of these tissue samples, ADRA1B promoter methylation was limited to Region 1 and/or Region 4, and no methylation was found in either Region 2 or Region 3. Thus, although one of 12 (8.3%) gastric cancers with reduced ADRA1B expression had no ADRA1B promoter methylation or 5q LOH, most cases with reduced ADRA1B expression had promoter methylation and 5q LOH; methylation in Region 2 and Region 3 strongly correlated with inactivation of ADRA1B expression.
Figure 4.
ADRA1B expression in gastric cancers was examined by RT–PCR. In this series, samples of cancer tissue from Case 1 and Case 2 and samples of surrounding epithelial tissue from Case 1 had markedly reduced ADRA1B expression as compared with that of the surrounding epithelial tissue of Case 2. As shown in Table 5, the samples of cancer tissue from Case 1 and Case 2 and of surrounding epithelial tissue from Case 1 exhibited ADRA1B promoter methylation in Region 2 and/or Region 3. No ADRA1B promoter methylation was found in the surrounding epithelial tissue of Case 2.
Table 5. Correlation among ADRA1B promoter methylationa, 5q LOH, and ADRA1B.
|
Cancer
|
Surrounding epithelial tissue
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case region | 1 | 2 | 3 | 4 | Expression | 5q LOH | Case region | 1 | 2 | 3 | 4 | Expression |
| 1 | M | U | M | M | Down | Negative | 1 | U | U | M | M | Down |
| 2 | U | M | M | U | Down | Negative | 2 | U | U | U | U | Normal |
| 3 | U | U | U | M | Normal | Negative | 3 | U | U | U | M | Normal |
| 4 | U | U | U | U | Normal | Negative | 4 | U | U | U | U | Normal |
| 5 | M | M | M | M | Down | Negative | 5 | U | U | U | M | Normal |
| 6 | U | M | U | U | Down | Positive | 6 | U | U | U | U | Normal |
| 7 | U | U | M | M | Down | Negative | 7 | U | U | U | U | Normal |
| 8 | U | U | U | M | Normal | Negative | 8 | U | U | U | U | Normal |
| 9 | M | M | M | M | Down | Negative | 9 | M | M | M | M | Down |
| 10 | U | U | U | U | Normal | Negative | 10 | U | U | U | U | Normal |
| 11 | U | U | U | U | Normal | Negative | 11 | U | U | U | U | Normal |
| 12 | U | M | M | M | Down | Negative | 12 | U | U | U | M | Normal |
| 13 | M | M | M | M | Down | Negative | 13 | U | M | M | M | Down |
| 14 | U | U | U | M | Normal | Negative | 14 | U | U | U | U | Normal |
| 15 | U | U | U | U | Normal | Negative | 15 | U | U | U | U | Normal |
| 16 | M | M | M | U | Down | Positive | 16 | U | U | U | U | Normal |
| 17 | U | U | M | M | Down | Positive | 17 | U | U | U | U | Normal |
| 18 | M | U | U | M | Normal | Negative | 18 | U | U | M | M | Normal |
Expression in 18 paired samples of gastric cancer and surrounding epithelial tissue.
Methylation status of the ADRA1B promoter in each region is represented as M or U. M indicates methylation-positive, and U indicates methylation-negative (unmethylated).
ADRA1B=alpha-1B-adrenergic receptor; LOH=loss of heterozygosity.
DISCUSSION
We applied the MS-RDA method to two paired samples of colorectal cancer and surrounding epithelial tissue, and identified ADRA1B. The ADRA1B promoter was aberrantly methylated in a subset of colorectal cancers, but not in corresponding epithelial tissues, suggesting that such methylation is a cancer-specific event during colorectal carcinogenesis. Furthermore, in gastric cancers, the ADRA1B promoter was very frequently methylated, generally in association with reduced ADRA1B expression. Alpha1-adrenergic receptors are members of the superfamily of G protein-coupled receptors and mediate effects related to the regulation of cellular hypertrophy and proliferation (Cruise et al, 1985; Lefkowitz and Caron, 1988; Cotecchia et al, 1990; Thonberg et al, 1994; Spector et al, 1997). Three distinct subtypes of alpha1-adrenergic receptors (alpha1A- (ADRA1A), alpha1B- (ADRA1B), and alpha1D- (ADRA1D) adrenergic receptors) have a prominent role in cell growth, and activation of ADRA1A and ADRA1B inhibits serum-prompted cell proliferation (Auer et al, 1998; Shibata et al, 2003). ADRA1B can activate the cyclin-dependent kinase inhibitors p27KIP1 and p21Cip1/WAF1, thereby inhibiting cell proliferation through this pathway (Auer et al, 1998; Shibata et al, 2003). Reduced ADRA1B expression might thus disrupt this pathway, giving cells aberrant growth advantage.
Our results first demonstrated ADRA1B promoter methylation in colorectal and gastric cancers. Alpha-1B-adrenergic receptor promoter methylation was particularly frequent in gastric cancers, whereas it was infrequent in colorectal cancers. Kim et al (2004) reported that 60% of gastric cancer cell lines and 64% of gastric cancers were methylated at the RUNX3 promoter, while RUNX3 promoter methylation was detected in only 4.9% colorectal cancers. Thus, the frequency of promoter methylation of a given tumour suppressor gene appears to vary among different types of cancer. Our results suggest that ADRA1B promoter methylation plays an important role in gastric cancer, but not in colorectal cancer, similar to RUNX3 promoter methylation. Furthermore, RT–PCR detected reduced ADRA1B expression in 12 of 18 (66.7%) gastric cancers, 11 (91.7%) of which concurrently had ADRA1B promoter methylation in Region 2 and/or Region 3. These results clearly suggested that ADRA1B promoter methylation is the principal mechanism for gene inactivation, with methylation of Region 2 and Region 3 (−517 to −213 relative to the transcription start site) in the promoter being especially critical. Three of 11 (27.3%) gastric cancers with reduced ADRA1B expression also had 5q LOH. Therefore, 5q LOH is apparently related to reduced ADRA1B expression in a subset of gastric cancers.
We also demonstrated that ADRA1B promoter methylation occurred in the surrounding epithelial tissues of gastric cancers, a small fraction of which concurrently had reduced ADRA1B expression. Several studies have shown that promoter methylation of multiple tumour-related genes is present in gastric epithelial tissues with or without cancer, and that accumulations of such genes promote gastric carcinogenesis (Leung et al, 1999; Kang et al, 2000; Kang et al, 2001, 2003a, 2003b; To et al, 2002; Waki et al, 2002; Chan et al, 2003). Waki et al (2002) reported that among 94 samples obtained from patients with gastric cancer, promoter methylation of E-cadherin, hMLH1, and p16 was found in 67, 24, and 44% of the surrounding gastric epithelial tissues, respectively. Kang et al (2003a) reported that among 268 samples obtained from gastric epithelial tissues without cancer, promoter methylation of DAP-kinase, E-cadherin, THBS1, TIMP3, p14, MGMT, p16, COX2, GSTP1 hMLH1, and RASSF1A was observed in 53.7, 41.0, 37.7, 23.1, 18.7, 10.9, 10.0, 4.1, 3.4, 1.7, and 0.4%, respectively. Our results demonstrated that ADRA1B promoter methylation was present in 14 of 34 (41.2%) surrounding epithelial tissues of gastric cancers; this frequency was similar to those of the important tumour-related genes mentioned above. Kang et al (2003b) also demonstrated that the average number of methylated genes markedly increases from non-neoplastic mucosa to intestinal-type gastritis. Although still controversial, the precancerous nature of IM has been suggested by the clinical phenomenon that gastric cancer often arises from intestinal-type gastritis. Although the difference did not reach statistical significance, the frequency of ADRA1B promoter methylation in surrounding epithelial tissues with IM was higher than that in surrounding epithelial tissues without IM. Thus, ADRA1B promoter methylation may also participate in the early phase of gastric carcinogenesis, similar to the other tumour-related genes mentioned above (Stemmermann, 1994). The degree of promoter methylation and the frequency of reduced ADRA1B expression in cancer are more extensive and more frequent than those in the surrounding epithelial tissues. With gastric carcinogenesis, ADRA1B promoter methylation spreads extensively, leading to reduced ADRA1B expression. Such reduced expression gives cell aberrant growth potential, resulting from loss of the growth inhibitory activity of ADRA1B.
In conclusion, our study showed that ADRA1B promoter is aberrantly hypermethylated in colorectal and gastric cancers. In gastric cancer, ADRA1B promoter methylation occurs frequently in both cancer tissue as well as in surrounding epithelial tissue. Our results suggest that ADRA1B is an important tumour-related gene with key roles in the development and progression of gastric cancer.
References
- Asada K, Miyamoto K, Fukutomi T, Tsuda H, Yagi Y, Wakazono K, Oishi S, Fukui H, Sugimura T, Ushijima T (2003) Reduced expression of GNA11 and silencing of MCT1 in human breast cancers. Oncology 64: 380–388 [DOI] [PubMed] [Google Scholar]
- Auer KL, Spector MS, Tombes RM, Seth P, Fisher PB, Gao B, Dent P, Kunos G (1998) α-Adrenergic inhibition of proliferation in HepG2 cells stably transfected with the α1B-adrenergic receptor through a p42MAPkinase/p21Cip1/WAF1-dependent pathway. FEBS Lett 436: 131–138 [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG (2000) DNA hypermethylation in tumoriogenesis : epigenesis joins genetics. Trends Genet 16: 168–174 [DOI] [PubMed] [Google Scholar]
- Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ, Kim HR, Chi SG (2003) Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinoma. Cancer Res 63: 7068–7075 [PubMed] [Google Scholar]
- Chan AOO, Lam SK, Wong BCY, Wong WM, Yuen MF, Yueng YH, Hui WM, Rashid A, Kwong YL (2003) Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 52: 502–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ASW, Tsui WY, Chen X, Chu KM, Chan TL, Chan ASY, Li R, So S, Yuen ST, Leung SY (2003) Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. Oncogene 22: 6946–6953 [DOI] [PubMed] [Google Scholar]
- Choi MC, Jong HS, Kim TY, Song SH, Lee DS, Lee JW, Kim TY, Kim NK, Bang YJ (2004) AKAP12/Gravin is inactivated by epigenetic mechanism in human gastric carcinoma and shows growth suppressor activity. Oncogene 23: 7095–7103 [DOI] [PubMed] [Google Scholar]
- Cotecchia S, Exum S, Caron MG, Lefkowitz RJ (1990) Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci USA 87: 2896–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise JL, Houck KA, Michalopoulos GK (1985) Induction of DNA synthesis in cultured rat hepatocytes through stimulation of alpha 1 adrenoreceptor by norepinephrine. Science 227: 749–751 [DOI] [PubMed] [Google Scholar]
- Esteller M, Corn PG, Baylin SB, Herman JG (2001) A gene hypermethylation profile of human cancer. Cancer Res 61: 3225–3229 [PubMed] [Google Scholar]
- Feinberg AP, Gehrke CW, Kuo KC, Ehrlich M (1988) Reduced genomic 5-methylcitosine content in human neoplasia. Cancer Res 48: 1159–1161 [PubMed] [Google Scholar]
- Fleisher AS, Esteller M, Wang S, Tamura G, Suzuki H, Yin J, Zou TT, Abraham JM, Kong D, Smolinski KN, Shi YQ, Ryu MG, Powell SM, James SP, Wilson KT, Herman JG, Meltzen SJ (1999) Hypermethylation of the hMLH1 gene promoter in human gastric cancers with microsatellite instability. Cancer Res 59: 1090–1095 [PubMed] [Google Scholar]
- Fuchs CS, Mayer RJ (1995) Gastric carcinoma. N Eng J Med 333: 32–41 [DOI] [PubMed] [Google Scholar]
- Gama-Sosa MA, Slagel VA, Trewyn RW, Oxenhandler R, Kuo KC, Gehrke CW, Ehrlich M (1983) The 5-methylcytosine content of DNA from human tumors. Nucleic Acid Res 11: 6833–6894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93: 821–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DSR, Gnarra JR, Linehan WM, Baylin SB (1994) Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 91: 9700–9704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda A, Kaminishi M, Nakanishi Y, Sugimura T, Ushijima T (2002a) Reduced expression of the insulin-induced protein 1 and p41APR2/3 complex genes in human gastric cancers. Int J Cancer 100: 57–62 [DOI] [PubMed] [Google Scholar]
- Kaneda A, Kaminishi M, Yanagihara K, Sugimura T, Ushijima T (2002b) Identification of silencing of nine genes in human gastric cancers. Cancer Res 62: 6645–6650 [PubMed] [Google Scholar]
- Kang GH, Lee HJ, Hwang KS, Lee S, Kim JH, Kim JS (2003a) Aberrant CpG island hypermethylation of chronic gastritis, in relation to aging, gender, intestinal metaplasia, and chronic inflammation. Am J Pathol 163: 1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang GH, Lee S, Kim JS, Jung HY (2003b) Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest 83: 635–641 [DOI] [PubMed] [Google Scholar]
- Kang GH, Shim YH, Jung HY, Kim WH, Ro JY, Rhyu MG (2001) CpG island methylation in premalignant stages of gastric carcinoma. Cancer Res 61: 2847–2851 [PubMed] [Google Scholar]
- Kang SH, Choi HH, Kim SG, Jong HS, Kim NK, Kim SJ, Bang YJ (2000) Transcriptional inactivation of the tissue inhibitor of metalloproteinase -3 gene by DNA hypermethylation of the 5′-CpG island in human gastric cancer cell lines. Int J Cancer 86: 632–635 [DOI] [PubMed] [Google Scholar]
- Kim TY, Lee HJ, Hwang KS, Lee M, Kim JW, Bang YJ, Kang GH (2004) Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest 84: 479–484 [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Caron MG (1988) Adrenergic receptors. Models for the study of receptors coupled to guanine nucleotide regulatory proteins. J Biol Chem 263: 4993–4996 [PubMed] [Google Scholar]
- Leung SY, Yuen ST, Chung LP, Chu KM, Chan ASY, Ho JCI (1999) hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 59: 159–164 [PubMed] [Google Scholar]
- Lueung WK, Yu J, Ng EKW, To KF, Ma PK, Lee TL, Go MYY, Chung SCS, Sung JJY (2001) Concurrent hypermethylation of multiple tumor-related genes in gastric carcinoma and adjacent normal tissues. Cancer 91: 2294–2301 [PubMed] [Google Scholar]
- Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB, Kim HM, Kim WJ, Yamamoto H, Yamashita N, Yano T, Ikeda T, Itohara S, Inazawa J, Abe T, Hagiwara A, Yamagishi H, Ooe A, Kaneda A, Sugimura T, Ushijima T, Bae S, Ito Y (2002) Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell 109: 113–124 [DOI] [PubMed] [Google Scholar]
- Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D (1995) 5′ CpG island methylation is associated with transcriptional silencing of the tumor suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1: 686–692 [DOI] [PubMed] [Google Scholar]
- Miotto E, Sabbioni S, Veronese A, Calin GA, Gullini S, Liboni A, Gramantieri L, Bolondi L, Ferrazzi E, Gafa R, Lanza G, Negrini M (2004) Frequent aberrant methylation of the CDH4 gene promoter in human colorectal and gastric cancer. Cancer Res 64: 8156–8159 [DOI] [PubMed] [Google Scholar]
- Ramarao CS, Denker JM, Perez DM, Gaivin RJ, Riek RP, Graham RM (1992) Genomic organization and expression of the human alpha 1B-adrenergic receptor. J Biol Chem 267: 21936–21945 [PubMed] [Google Scholar]
- Shibata K, Katsuma S, Koshimizu T, Shinoura H, Hirasawa A, Tanoue A, Tsujimoto G (2003) 1-Adrenergic receptor subtypes differentially control the cell cycle of trasfected CHO cells through a camp-dependent mechanism involving p27Kip1. J Biol Chem 273: 672–678 [DOI] [PubMed] [Google Scholar]
- Shim YH, Kang GH, Ro JY (2000) Correlation of p16 hypermethylation with the p16 protein loss in sporadic gastric carcinoma. Lab Invest 80: 689–695 [DOI] [PubMed] [Google Scholar]
- Spector MS, Auer KL, Jarvis WD, Ishac EJ, Gao B, Kunos G, Dent P (1997) Differential regulation of the mitogen-activated protein and stress-activated protein kinase cascades by adrenergic agonists in quiescent and regenerating adult rat hepatocytes. Mol Cell Biol 17: 3556–3565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmermann GN (1994) Intestinal metaplasia of the stomach. A status report. Cancer 74: 556–564 [DOI] [PubMed] [Google Scholar]
- Takai D, Yagi Y, Wakazono K, Ohishi N, Morita Y, Sugimura T, Ushijima T (2001) Silencing of HTR1B and reduced expression of EDN1 in human lung cancers, revealed by methylation-sensitive representational difference analysis. Oncogene 20: 7505–7513 [DOI] [PubMed] [Google Scholar]
- Thonberg H, Zhang SJ, Tvrdik P, Jacobsson A, Nedergaard J (1994) Norepinephrine utilizes alpha 1- and beta-adrenoreceptors synergistically to maximally induce c-fos expression in brown adipocytes. J Biol Chem 269: 33179–33186 [PubMed] [Google Scholar]
- To KF, Leung WK, Lee TL, Yu J, Tong JHM, Chan MWY, Ng EKW, Chung SCS, Sung JJY (2002) Promoter hypermethylation of tumor-related genes in gastric intestinal metaplasia of patients with and without gastric cancer. Int J Cancer 102: 623–628 [DOI] [PubMed] [Google Scholar]
- Tokumaru Y, Nomoto S, Jeronimo C, Henrique R, Harden S, Trink B, Shidransky D (2003) Bialleic inactivation of the RIZ1 gene in human gastric cancer. Oncogene 22: 6954–6958 [DOI] [PubMed] [Google Scholar]
- Toyota M, Ahuja N, Suzuki H, Itoh F, Ohe-Toyota M, Imai K, Baylin SB, Issa JP (1999) Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res 59: 5438–5442 [PubMed] [Google Scholar]
- Ushijima T, Morimura K, Hosoya Y, Okonogi H, Tatematsu M, Sugimura T, Nagao M (1997) Establishment of methylation-sensitive-representational difference analysis and isolation of hypo- and hypermethylated genomic fragments in mouse liver tumors. Proc Natl Acad Sci USA 94: 2284–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki T, Tamura G, Tsuchiya T, Sato K, Nishizuka S, Motoyama T (2002) Promoter methylation status of E-cadherin, hMLH1, and p16 genes in nonneoplastic gastric epithelia. Am J Pathol 161: 399–403 [DOI] [PMC free article] [PubMed] [Google Scholar]