Abstract
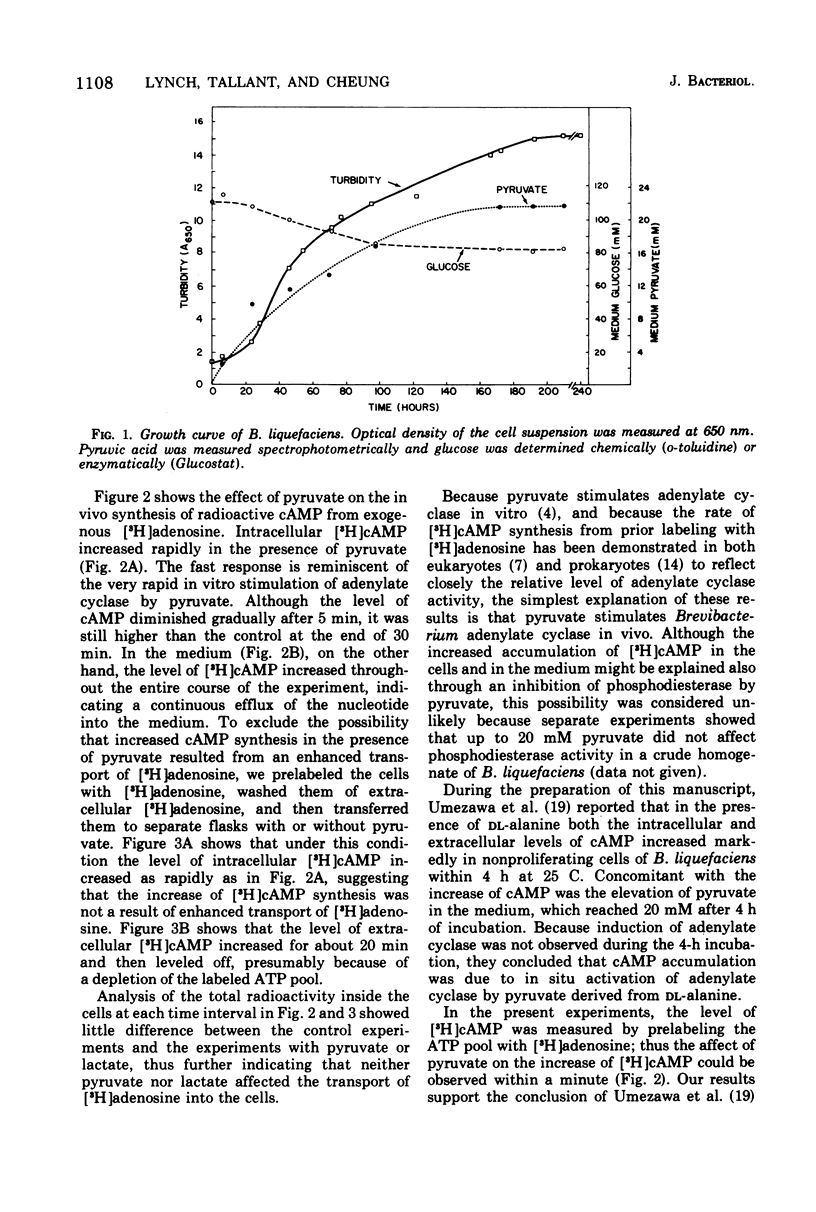
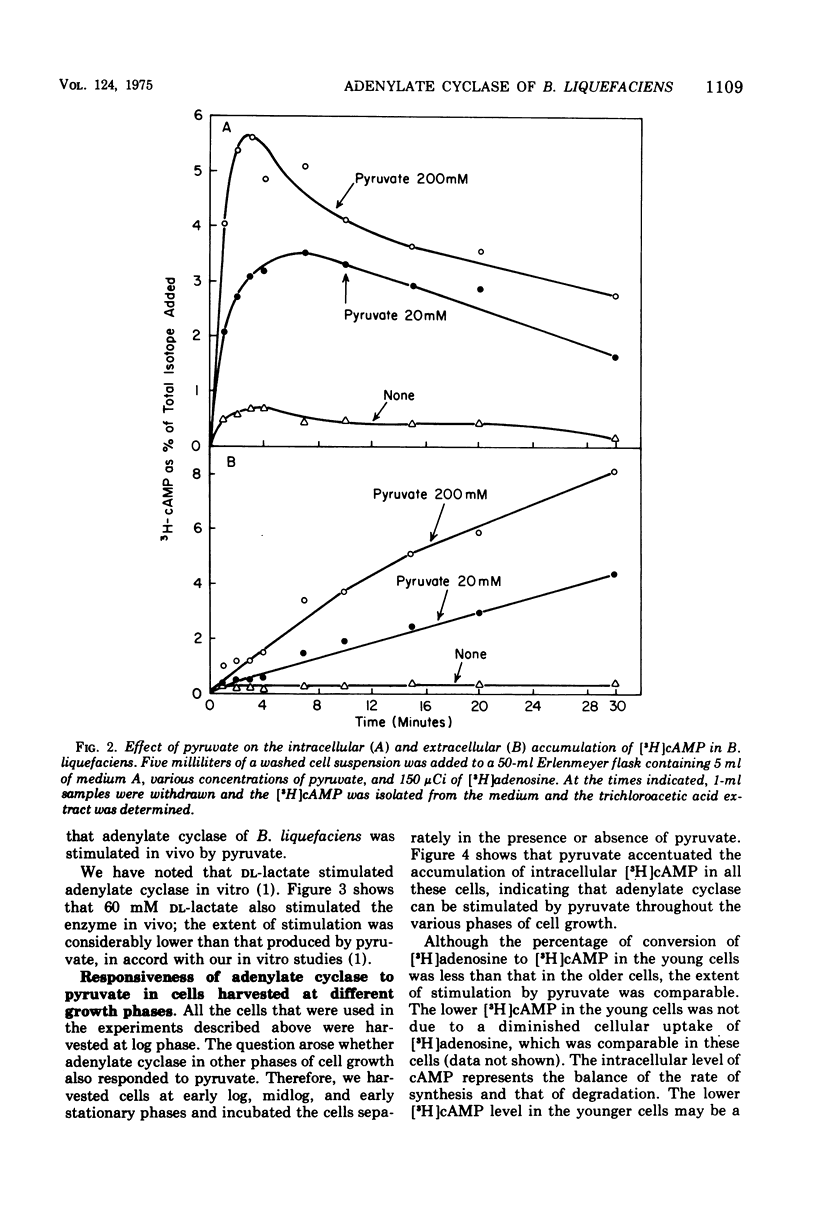
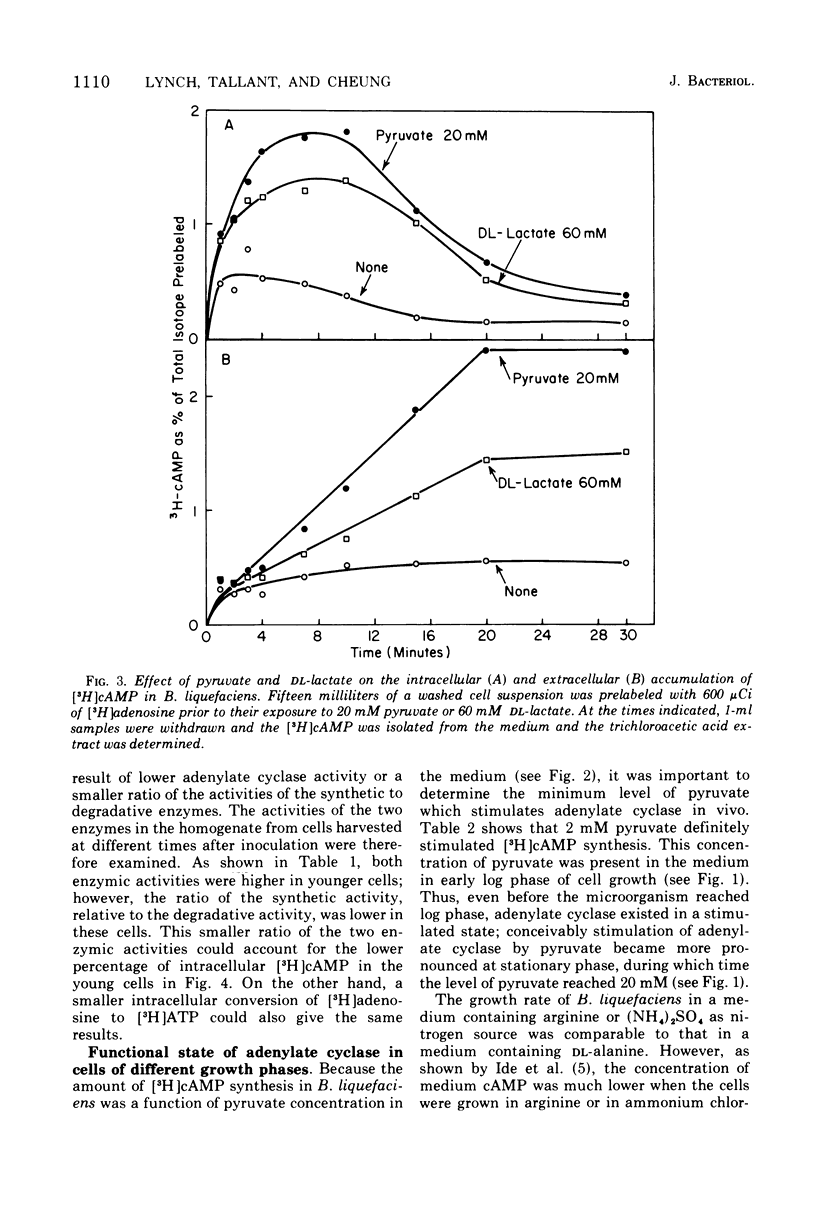
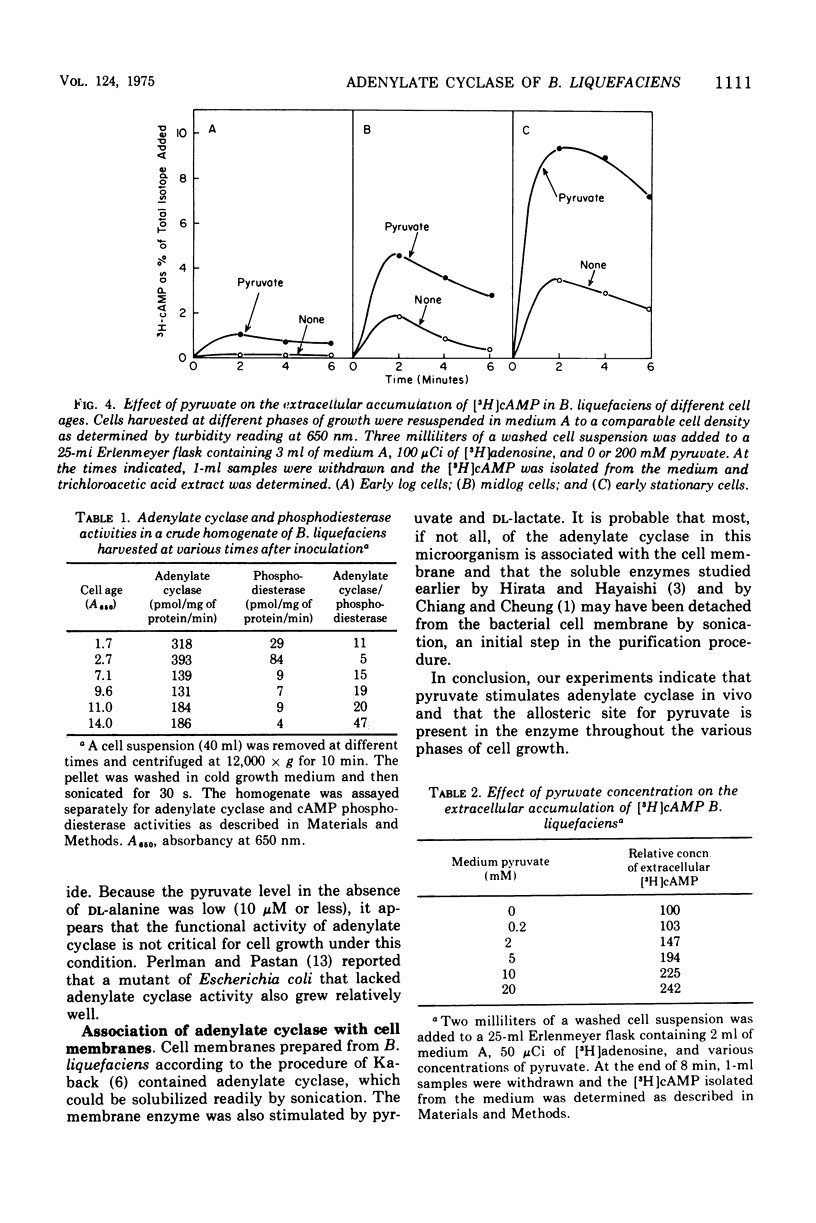
Adenylate cyclase of Brevibacterium liquefaciens depends on pyruvate for activity. Growing in a simple medium containing glucose and DL-alanine, the microorganism excreted pyruvate, which reached 20 mM in the medium at stationary phase. Using [3H]adenosine to label the adenosine 5'-triphosphate pool, we showed that pyruvate in the medium stimulated adenylate cyclase of B. liquefaciens in vivo, in a manner similar to the stimulation observed in vitro. Adenylate cyclase in cells harvested at different phases of growth was equally responsive to exogenous pyruvate, indicating that the allosteric site for pyruvate was present in the enzyme throughout the various phases of cell growth. The specific activity of adenylate cyclase was highest in cells harvested at early log phase; thereafter it declined and was substantially lower at stationary phase. Although adenylate cyclase appears to be activated by pyruvate throughout the life span of the cell, the activity appears not to be critical to cell growth, which was comparable whether the medium contained high or low pyruvate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiang M. H., Cheung W. Y. Adenylate cyclase of Brevibacterium liquefaciens. Inactivation by neuraminidase, phospholipase A and phospholipase C. Biochem Biophys Res Commun. 1973 Nov 1;55(1):187–192. doi: 10.1016/s0006-291x(73)80077-4. [DOI] [PubMed] [Google Scholar]
- DUBOWSKI K. M. An o-toluidine method for body-fluid glucose determination. Clin Chem. 1962 May-Jun;8:215–235. [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Adenyl cyclase of Brevibacterium liquefaciens. Biochim Biophys Acta. 1967 Nov 21;149(1):1–11. doi: 10.1016/0005-2787(67)90685-5. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayaishi O. Pyruvate dependent adenyl cyclase activity of Brevibacterium liquefaciens. Biochem Biophys Res Commun. 1965 Nov 22;21(4):361–365. doi: 10.1016/0006-291x(65)90202-0. [DOI] [PubMed] [Google Scholar]
- Ide M., Yoshimoto A., Okabayashi T. Formation of adenosine cyclic 3',5'-phosphate by nonproliferating cells and cell-free extract of Brevibacterium liquefaciens. J Bacteriol. 1967 Aug;94(2):317–322. doi: 10.1128/jb.94.2.317-322.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian J. W., Kuo J. F., Greengard P. Determination of relative levels of cyclic AMP in tissues or cells prelabeled with radioactive adenine. Adv Cyclic Nucleotide Res. 1972;2:131–137. [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Kuo J. F., De Renzo E. C. A comparison of the effects of lipolytic and antilipolytic agents on adenosine 3',5'-monophosphate levels in adipose cells as determined by prior labeling with adenine-8-14C. J Biol Chem. 1969 May 10;244(9):2252–2260. [PubMed] [Google Scholar]
- Kurashina Y., Takai K., Suzuki-Hori C., Okamoto H., Hayaishi O. Adenylate cyclase from Brevibacterium liquefaciens. Reversibility and thermodynamic studies. J Biol Chem. 1974 Aug 10;249(15):4824–4828. [PubMed] [Google Scholar]
- Lynch T. J., Cheung W. Y. Underestimation of cyclic 3',5'-nucleotide phosphodiesterase activity by a radioisotopic assay using an anionic-exchange resin. Anal Biochem. 1975 Jul;67(1):130–138. doi: 10.1016/0003-2697(75)90280-8. [DOI] [PubMed] [Google Scholar]
- OKABAYASHI T., YOSHIMOTO A., IDE M. OCCURRENCE OF NUCLEOTIDES IN CULTURE FLUIDS OF MICROORGANISMS. V. EXCRETION OF ADENOSINE CYCLIC 3',5'-PHOSPHATE BY BREVIBACTERIUM LIQUEFACIENS SP. N. J Bacteriol. 1963 Nov;86:930–936. doi: 10.1128/jb.86.5.930-936.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman R. L., Pastan I. Pleiotropic deficiency of carbohydrate utilization in an adenyl cyclase deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1969 Sep 24;37(1):151–157. doi: 10.1016/0006-291x(69)90893-6. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Measurements of rates of adenosine 3':5'-cyclic monophosphate synthesis in intact Escherichia coli B. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2149–2152. doi: 10.1073/pnas.70.7.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Schönhöfer P. S., Skidmore I. F. Studies on the conditions for cyclic AMP formation in homogenised and intact fat cells by use of 3 H-ATP and 3 H-adenine. Pharmacology. 1971;6(2):109–125. doi: 10.1159/000136232. [DOI] [PubMed] [Google Scholar]
- Takai K., Kurashina Y., Suzuki-Hori C., Okamoto H., Hayaishi O. Adenylate cyclase from Brevibacterium liquefaciens. I. Purification, crystallization, and some properties. J Biol Chem. 1974 Mar 25;249(6):1965–1972. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]
- Umezawa K., Takai K., Tsuji S., Kurashina Y., Hayaishi O. Adenylate cyclase from Brevibacterium liquefaciens. III. In situ regulation of adenylate cyclase by pyruvate. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4598–4601. doi: 10.1073/pnas.71.11.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]


