Abstract
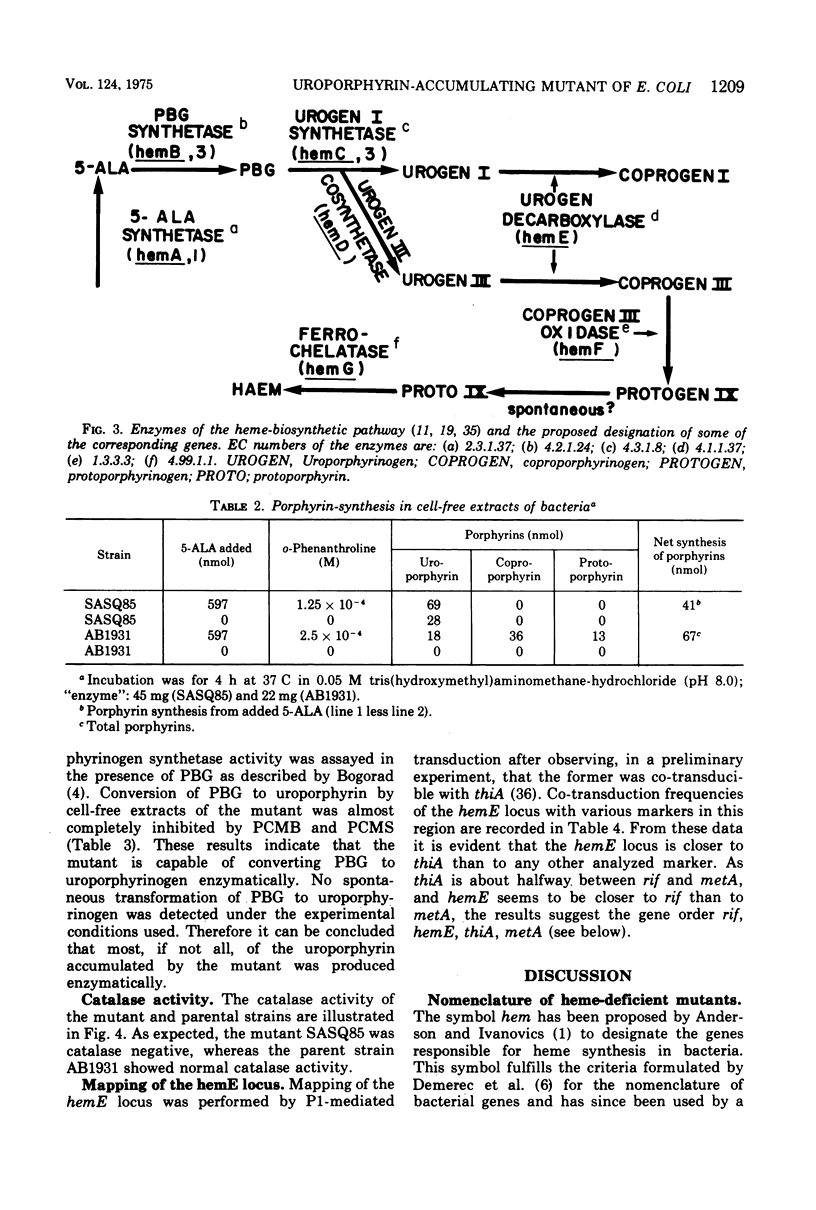
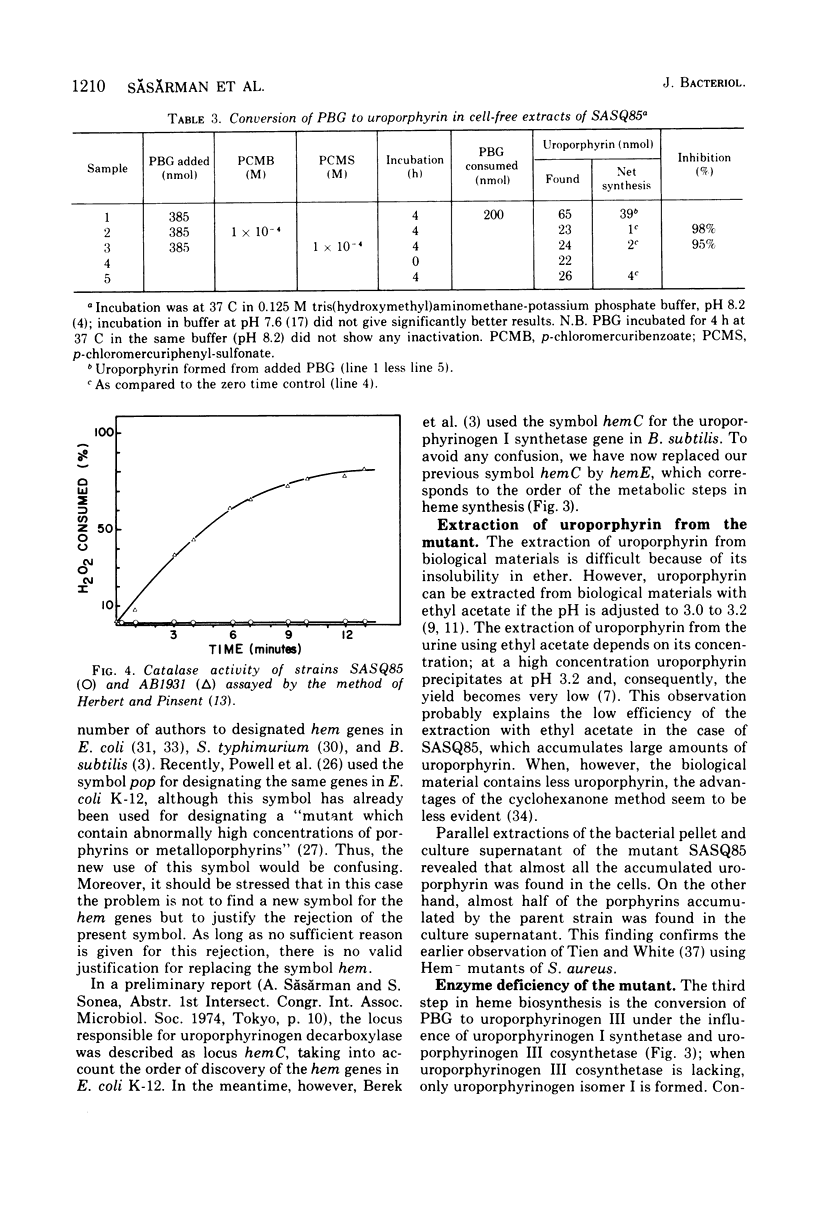
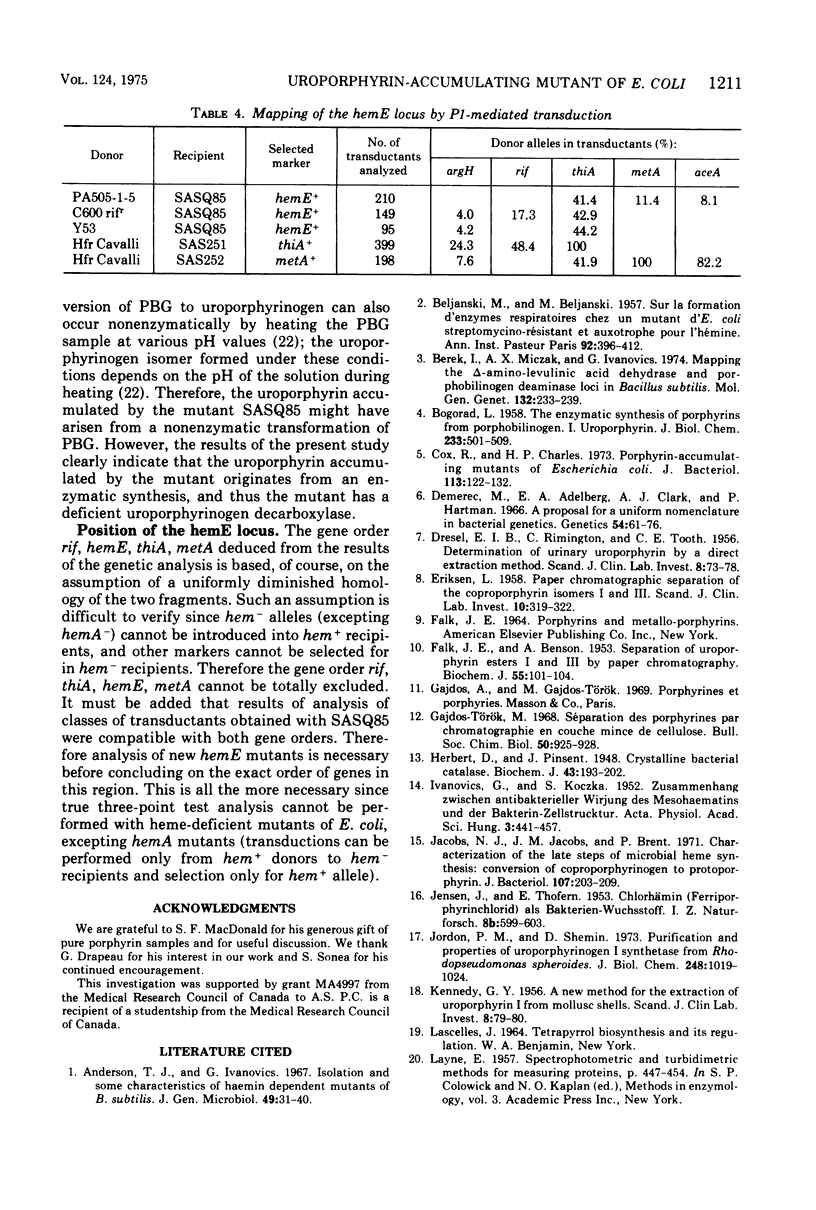
An uroporphyrin III-accumulating mutant of Escherichia coli K-12 was isolated by neomycin. The mutant, designated SASQ85, was catalase deficient and formed dwarf colonies on usual media. Comparative extraction by cyclohexanone and ethyl acetate showed the superiority of the former for the extraction of the uroporphyrin accumulated by the mutant. Cell-free extracts of SASQ85 were able to convert 5-aminolevulinic acid and porphobilinogen to uroporphyrinogen, but not to copro- or protoporphyrinogen. Under the same conditions cell-free extracts of the parent strain converted 5-aminolevulinic to uroporphyringen, coproporphyrinogen, and protoporphyrinogen. The conversion of porphobilinogen to uroporphyrinogen by cell-free extracts of the mutant was inhibited 98 and 95%, respectively, by p-chloromercuribenzoate and p-chloromercuriphenyl-sulfonate, indicating the presence of uroporphyrinogen synthetase activity in the extracts. Spontaneous transformation of porphobilinogen to uroporphyrin was not detectable under the experimental conditions used [4 h at 37 C in tris(hydroxymethyl)aminomethane-potassium phosphate buffer, pH 8.2]. The results indicate a deficient uroporphyrinogen decarboxylase activity of SASQ85 which is thus the first uroporphyrinogen decarboxylase-deficient mutant isolated in E. coli K-12. Mapping of the corresponding locus by P1-mediated transduction revealed the frequent joint transduction of hemE and thiA markers (frequency of co-transduction, 41 to 44%). The results of the genetic analysis suggest the gene order rif, hemE, thiA, metA; however, they do not totally exclude the gene order rif, thiA, hemE, metA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson T. J., Ivánovics G. Isolation and some characteristics of haemin dependent mutants of Bacillus subtilis. J Gen Microbiol. 1967 Oct;49(1):31–40. doi: 10.1099/00221287-49-1-31. [DOI] [PubMed] [Google Scholar]
- BELJANSKI M. Sur la formation d'enzymes respiratoires chez un mutant d'Escherichia coli streptomycino-résistant et auxotrophe pour l'hémine. Ann Inst Pasteur (Paris) 1957 Mar;92(3):396–412. [PubMed] [Google Scholar]
- BOGORAD L. The enzymatic synthesis of porphyrins from porphobilinogen. I. Uroporphyrin I. J Biol Chem. 1958 Aug;233(2):501–509. [PubMed] [Google Scholar]
- Berek I., Miczák A., Ivánovics G. Mapping the delta-aminolaevulinic acid dehydrase and porphobilinogen deaminase loci in Bacillus subtilis. Mol Gen Genet. 1974;132(3):233–239. doi: 10.1007/BF00269396. [DOI] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESEL E. I., RIMINGTON C., TOOTH B. E. Determination of urinary uroporphyrin by a direct extraction method. Scand J Clin Lab Invest. 1956;8(1):73–78. doi: 10.3109/00365515609049247. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERIKSEN L. Paper chromatographic separation of the coproporphyrin isomers I and III. Scand J Clin Lab Invest. 1958;10(3):319–322. [PubMed] [Google Scholar]
- FALK J. E., BENSON A. Separation of uroporphyrin esters I and III by paper chromatography. Biochem J. 1953 Aug;55(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Gajdos-Török M. Séparation des porphyrines par chromatographie en couche mince de cellulose. Bull Soc Chim Biol (Paris) 1968;50(4):925–928. [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVICS G., KOCZKA S. Zusammenhang zwischen Antibakterieller Wirkung des Mesohaematins und der Bakterien-Zellstruktur. Acta Physiol Acad Sci Hung. 1952;3(2):441–457. [PubMed] [Google Scholar]
- Jacobs N. J., Jacobs J. M., Brent P. Characterization of the late steps of microbial heme synthesis: conversion of coproporphyrinogen to protoporphyrin. J Bacteriol. 1971 Jul;107(1):203–209. doi: 10.1128/jb.107.1.203-209.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Shemin D. Purification and properties of uroporphyrinogen I synthetase from Rhodopseudomonas spheroides. J Biol Chem. 1973 Feb 10;248(3):1019–1024. [PubMed] [Google Scholar]
- KENNEDY G. Y. A new method for the extraction of uroporphyrin I from mollusc shells. Scand J Clin Lab Invest. 1956;8(1):79–80. doi: 10.3109/00365515609049248. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasarman A., Surdeanu M., Portelance V., Dobardzic R., Sonea S. Vitamin K-deficient mutants of bacteria. Nature. 1969 Oct 18;224(5216):272–272. doi: 10.1038/224272a0. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Horodniceanu T. Locus determining normal colony formation on the chromosome of Escherichia coli K-12. J Bacteriol. 1967 Oct;94(4):1268–1269. doi: 10.1128/jb.94.4.1268-1269.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Sanderson K. E., Surdeanu M., Sonea S. Hemin-deficient mutants of Salmonella typhimurium. J Bacteriol. 1970 May;102(2):531–536. doi: 10.1128/jb.102.2.531-536.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien W., White D. C. Linear sequential arrangement of genes for the biosynthetic pathway of protoheme in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1392–1398. doi: 10.1073/pnas.61.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D. E. The effects of ultraviolet light and ionizing radiation on the transduction of Escherichia coli by phage P1. Virology. 1960 Jul;11:533–546. doi: 10.1016/0042-6822(60)90098-2. [DOI] [PubMed] [Google Scholar]
- Wulff D. L. Delta-aminolevulinic acid-requiring mutant from Escherichia coli. J Bacteriol. 1967 Apr;93(4):1473–1474. doi: 10.1128/jb.93.4.1473-1474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


