Abstract
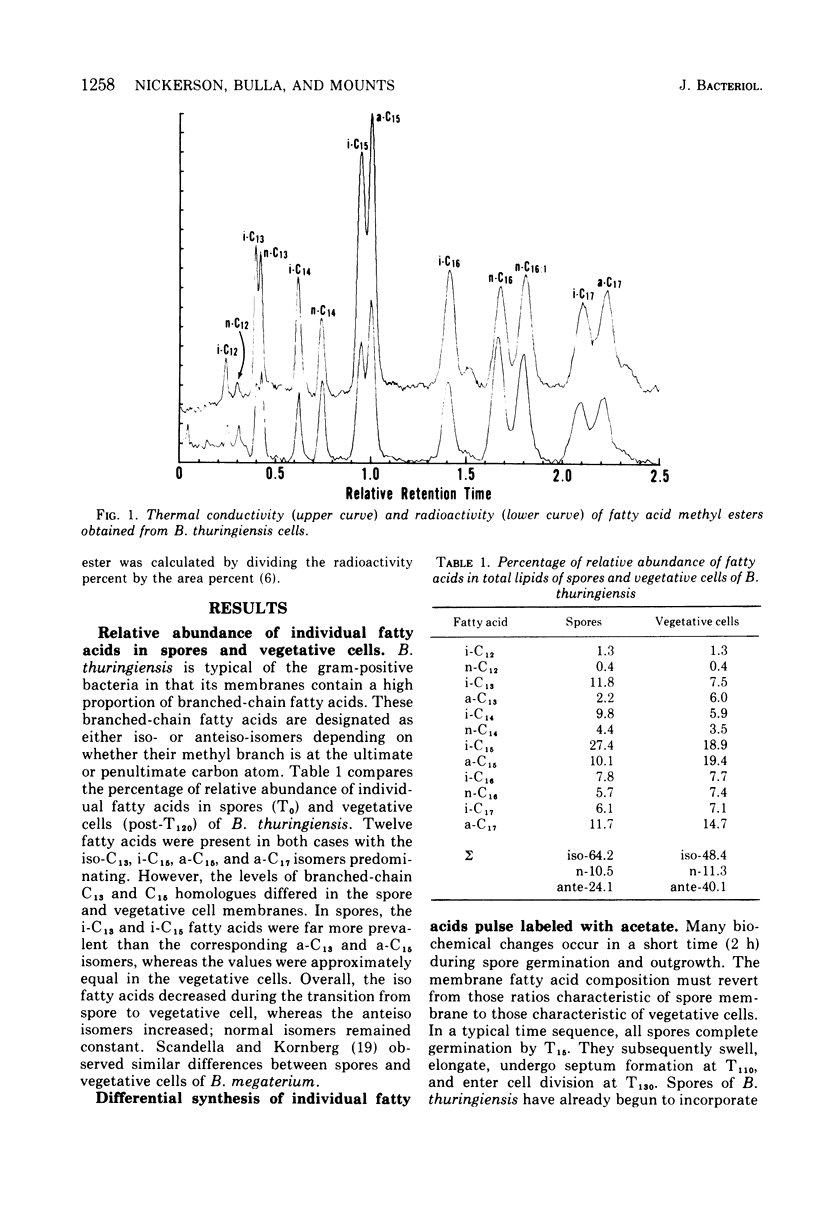
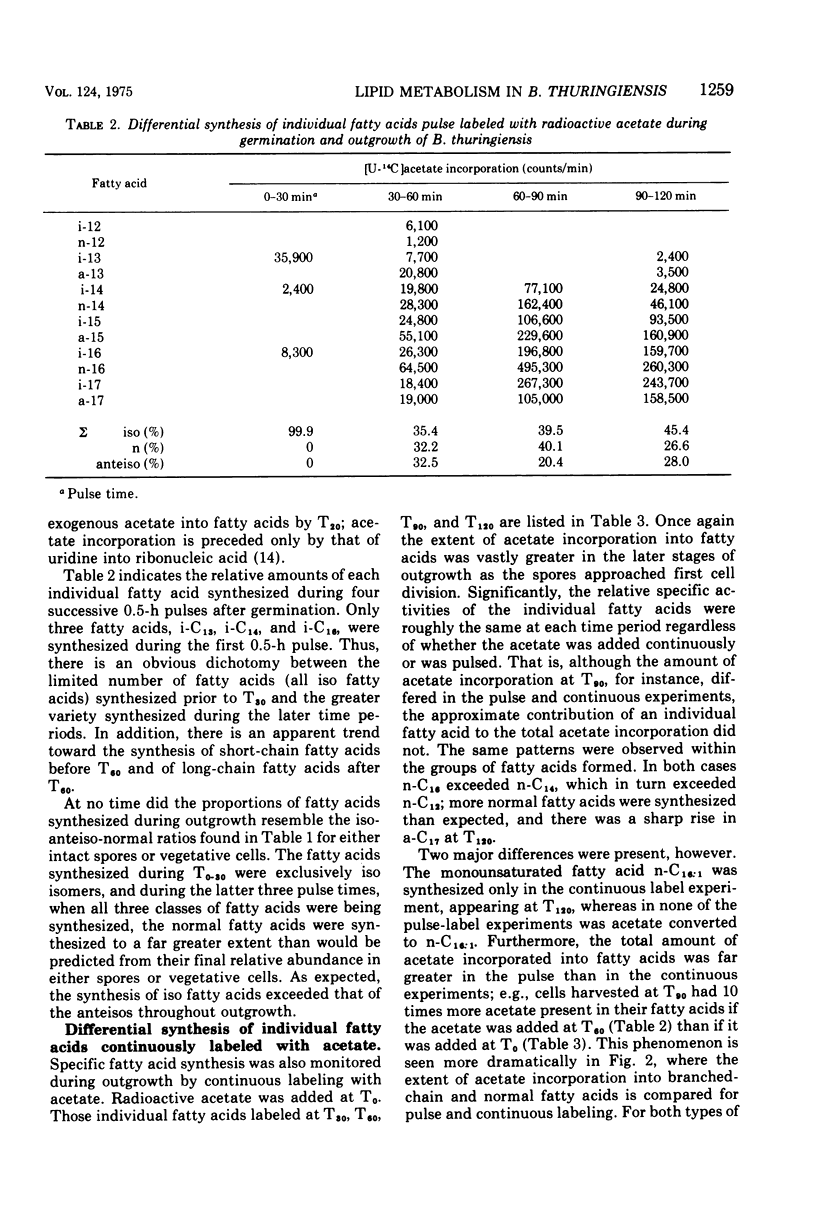
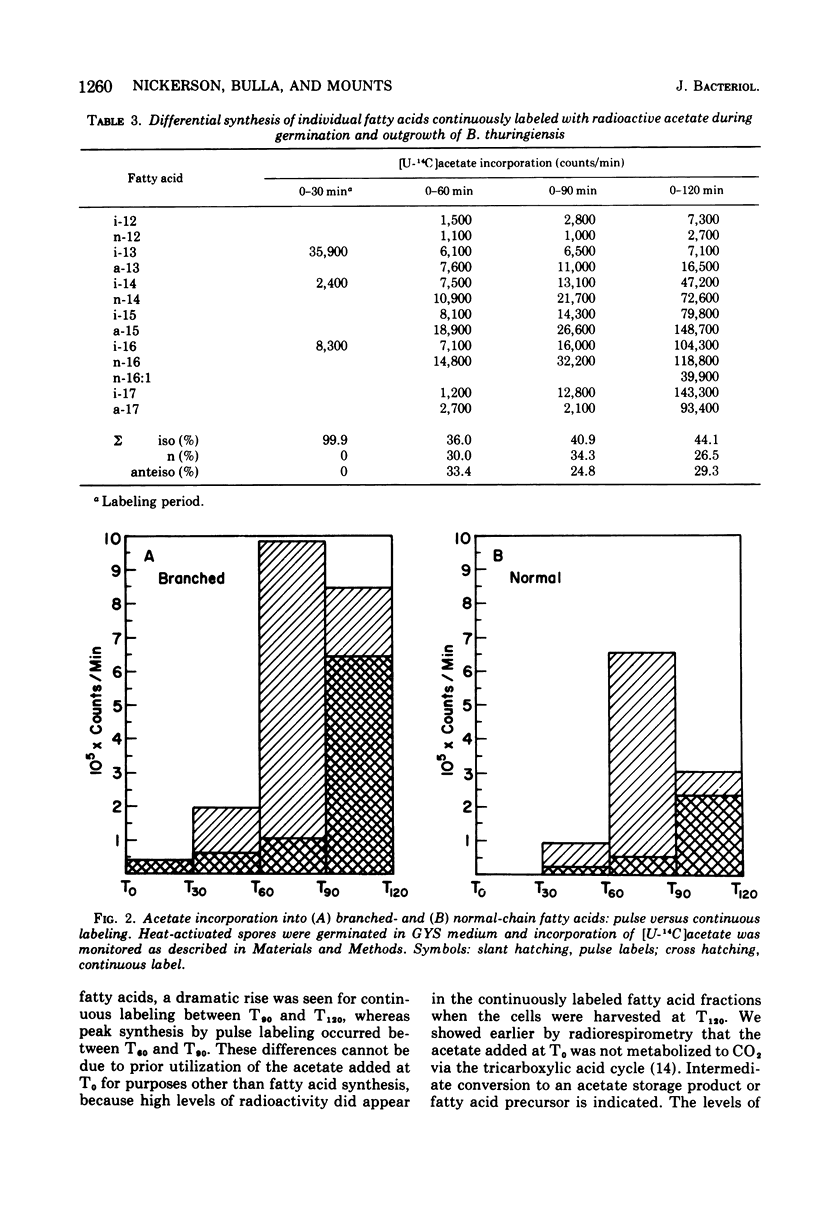
The biosynthesis of individual branched- and normal-chain fatty acids during Bacillus thuringiensis spore germination and outgrowth was studied by comparing pulsed and continuous labeling of these fatty acids with [U-14C]acetate. The relative specific activity of each fatty acid varies with time as the cell progresses through outgrowth. However, fatty acid synthesis does occur in two distinct phases. Upon germination, acetate is incorporated only into the iso-isomers i-C13, i-C14, and i-C16; no normal or anteiso synthesis occurs. Subsequent to T30, the full complement of branched- and normal-chain homologues is formed and there is a dramatic enhancement in the overall rate of fatty acid synthesis. Significantly, this rate increase coincides with a marked shift from the synthesis of short-chain to long-chain fatty acids. These findings illustrate a dichotomy in synthesis that may result from initial fatty acid formation by preexisting spore fatty acid biosynthetic enzymes in the absence of de novo protein synthesis. Elucidation of the timing and kinetics of individual fatty acid formation provides a biochemical profile of activities directly related to membrane differentiation and cellular development.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Rutberg L., Samuelsson B. The chemical composition of the cytoplasmic membrane of Bacillus subtilis. Eur J Biochem. 1967 Nov;2(4):448–453. doi: 10.1111/j.1432-1033.1967.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Bulla L. A., Bennett G. A., Shotwell O. L. Physiology of Sporeforming Bacteria Associated with Insects II. Lipids of Vegetative Cells. J Bacteriol. 1970 Dec;104(3):1246–1253. doi: 10.1128/jb.104.3.1246-1253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton H. J., Mounts T. L. Desaturation of fatty acids in seeds of higher plants. J Lipid Res. 1966 Mar;7(2):221–225. [PubMed] [Google Scholar]
- Fitz-James P. C. Formation of protoplasts from resting spores. J Bacteriol. 1971 Mar;105(3):1119–1136. doi: 10.1128/jb.105.3.1119-1136.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fralick J. A., Lark K. G. Evidence for the involvement of unsaturated fatty acids in initiating chromosome replication in Escherichia coli. J Mol Biol. 1973 Nov 5;80(3):459–475. doi: 10.1016/0022-2836(73)90416-6. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Biosynthesis of branched-chain fatty acids. IV. Factors affecting relative abundance of fatty acids produced by Bacillus subtilis. Can J Microbiol. 1966 Jun;12(3):501–514. doi: 10.1139/m66-073. [DOI] [PubMed] [Google Scholar]
- Kaneda T. Fatty acids in the genus Bacillus. II. Similarity in the fatty acid compositions of Bacillus thuringiensis, Bacillus anthracis, and Bacillus cereus. J Bacteriol. 1968 Jun;95(6):2210–2216. doi: 10.1128/jb.95.6.2210-2216.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson K. W., De Pinto J., Bulla L. A., Jr Lipid metabolism during bacterial growth, sporulation, and germination: kinetics of fatty acid and macromolecular synthesis during spore germination and outgrowth of Bacillus thuringiensis. J Bacteriol. 1975 Jan;121(1):227–233. doi: 10.1128/jb.121.1.227-233.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read B. D., McElhaney R. N. Glucose transport in Acholeplasma laidlawii B: dependence on the fluidity and physical state of membrane lipids. J Bacteriol. 1975 Jul;123(1):47–55. doi: 10.1128/jb.123.1.47-55.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Jacob F. DNA-membrane complex and nuclear segregation in bacteria. Cold Spring Harb Symp Quant Biol. 1968;33:669–676. doi: 10.1101/sqb.1968.033.01.076. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. Biochemical studies of bacterial sporulation and germination. XV. Fatty acids in growth, sporulation, and germination of Bacillus megaterium. J Bacteriol. 1969 Apr;98(1):82–86. doi: 10.1128/jb.98.1.82-86.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K. A., Kostiw L. L., Tyson B. J. Alterations in normal fatty acid composition in a temperature-sensitive mutant of a thermophilic bacillus. Arch Microbiol. 1974 Apr 19;97(2):89–102. doi: 10.1007/BF00403049. [DOI] [PubMed] [Google Scholar]
- Yousten A. A., Rogoff M. H. Metabolism of Bacillus thuringiensis in relation to spore and crystal formation. J Bacteriol. 1969 Dec;100(3):1229–1236. doi: 10.1128/jb.100.3.1229-1236.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


