Abstract
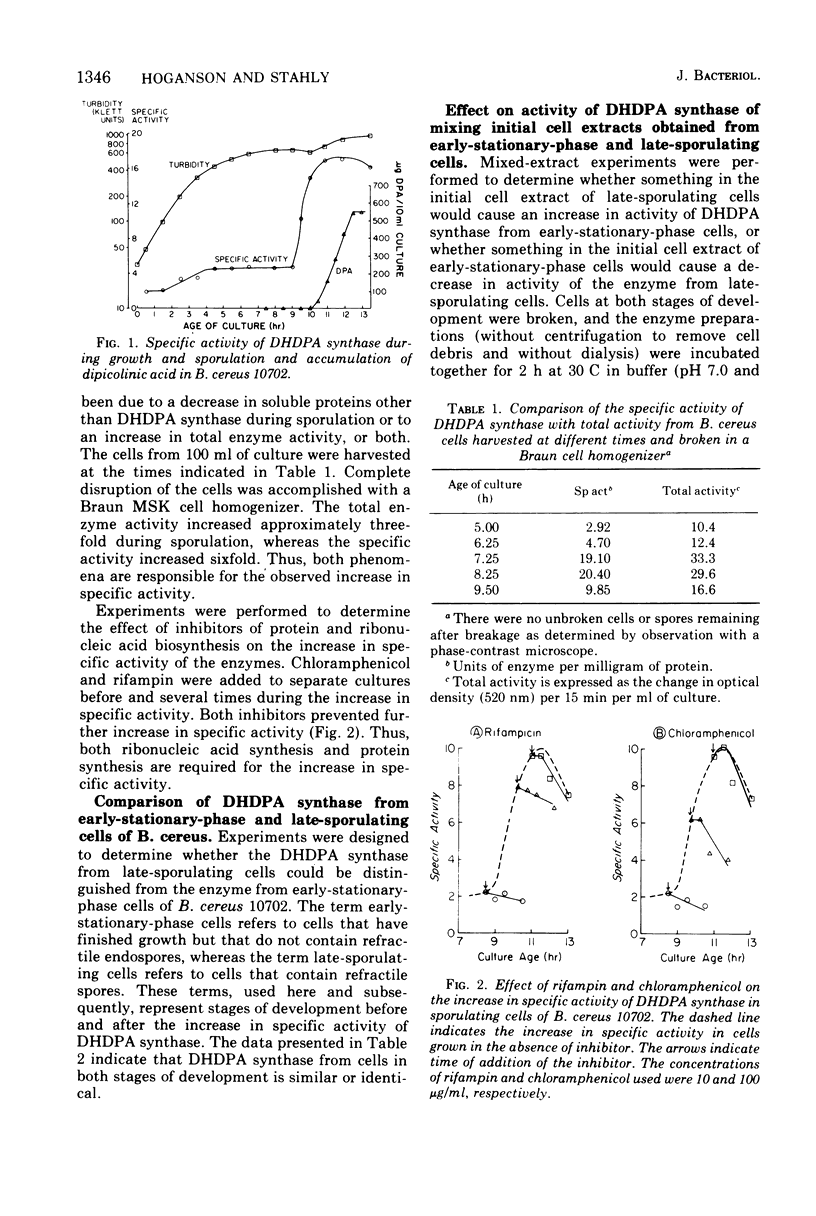
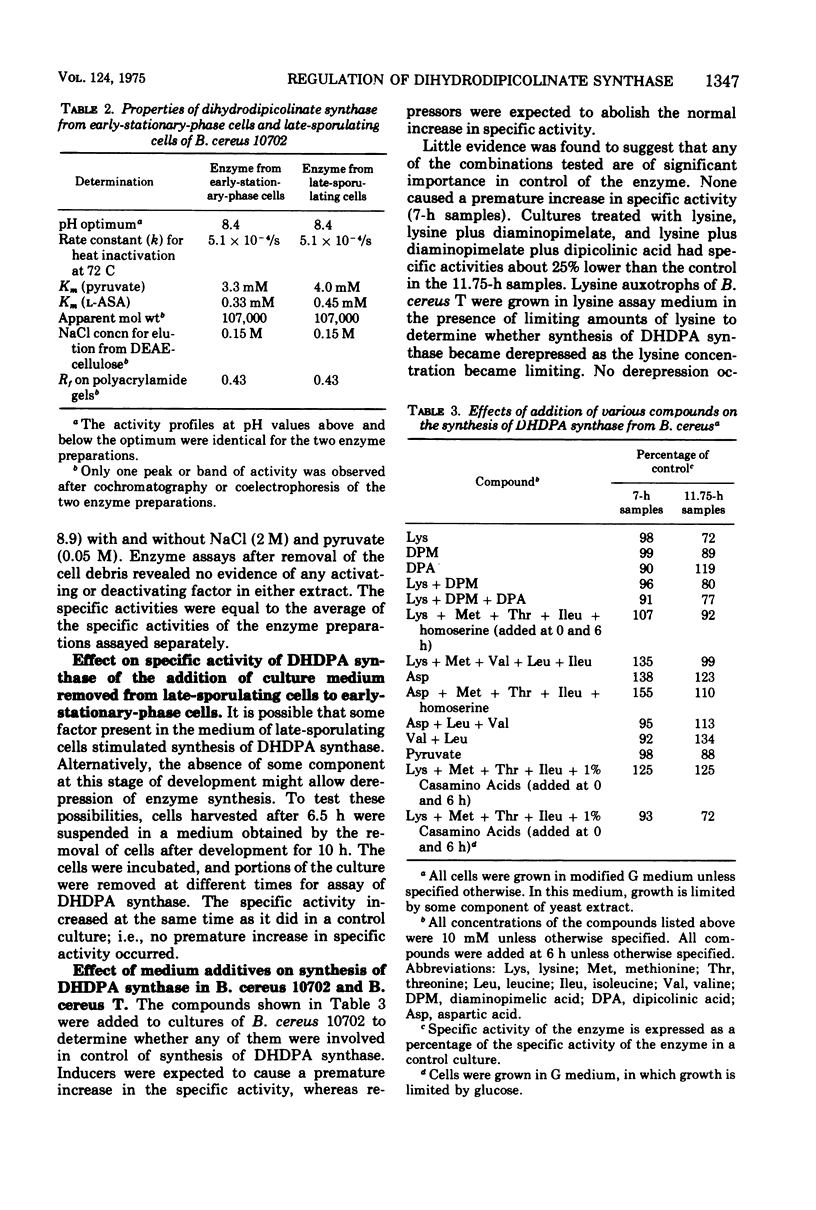
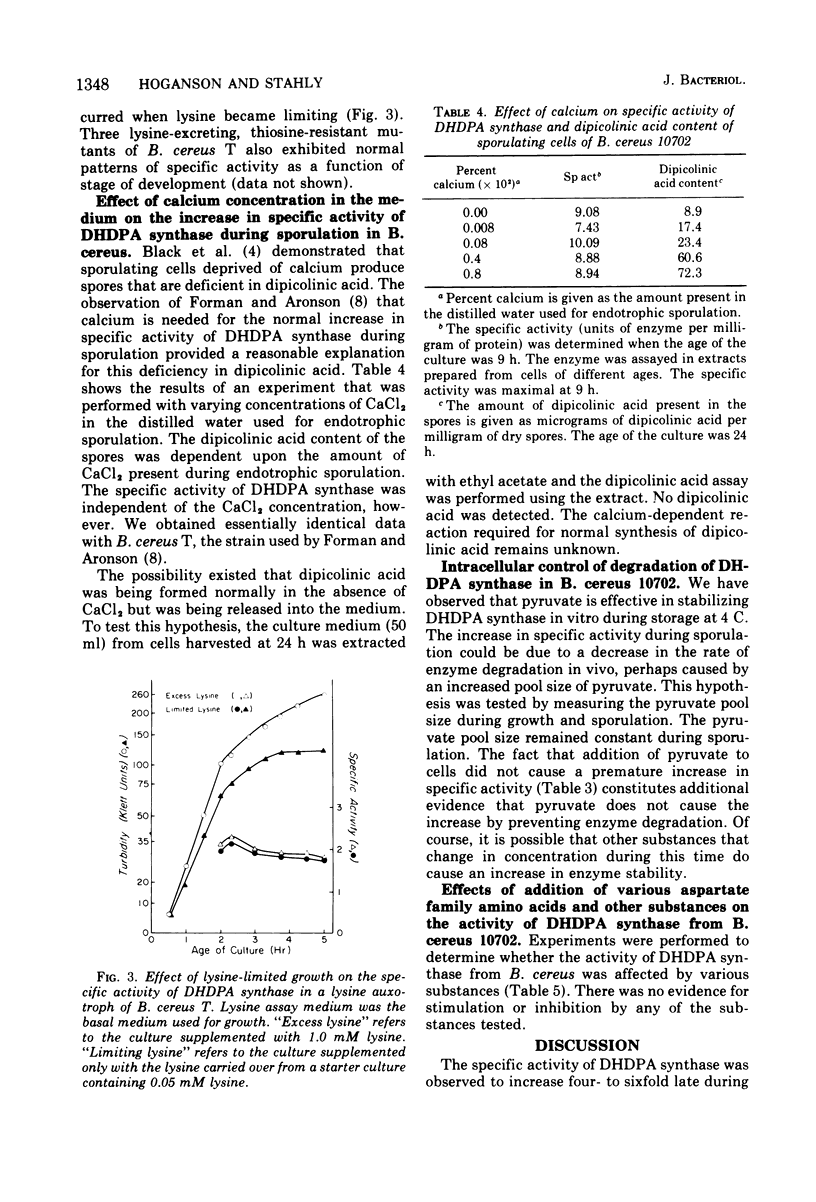
A four- to sixfold increase in specific activity of dihydrodipicolinic acid synthase was observed during sporulation of Bacillus cereus. The enzyme from cells harvested before and after the increase in specific activity appeared to be very similar as judged by pH optima, heat denaturation kinetics, apparent Michaelis constants, chromatography on diethylaminoethyl-cellulose and Sephadex G-200, and polyacrylamide gel electrophoresis. Studies with various combinations of amino acids and one of the enzyme substrates, pyruvate, failed to give evidence for control of the enzyme by activation, inhibition, repression, induction, or stabilization. Omission of calcium from the sporulation medium had no significant effect on the specific activity pattern of the enzyme as a function of age of culture.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Henderson E., Tincher A. Participation of the lysine pathway in dipicolinic acid synthesis in Bacillus cereus T. Biochem Biophys Res Commun. 1967 Feb 21;26(4):454–460. doi: 10.1016/0006-291x(67)90568-2. [DOI] [PubMed] [Google Scholar]
- BLACK S. H., HASHIMOTO T., GERHARDT P. Calcium reversal of the heat susceptibility and dipicolinate deficiency of spores formed "endotrophically" in water. Can J Microbiol. 1960 Apr;6:213–224. doi: 10.1139/m60-023. [DOI] [PubMed] [Google Scholar]
- BLACK S., WRIGHT N. G. Aspartic beta-semialdehyde dehydrogenase and aspartic beta-semialdehyde. J Biol Chem. 1955 Mar;213(1):39–50. [PubMed] [Google Scholar]
- Bach M. L., Gilvarg C. Biosynthesis of dipicolinic acid in sporulating Bacillus megaterium. J Biol Chem. 1966 Oct 10;241(19):4563–4564. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FOSTER J. W., PERRY J. J. Intracellular events occurring during endotrophic sporulation in Bacillus mycoides. J Bacteriol. 1954 Mar;67(3):295–302. doi: 10.1128/jb.67.3.295-302.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman M., Aronson A. Regulation of dipicolinic acid biosynthesis in sporulating Bacillus cereus. Characterization of enzymic changes and analysis of mutants. Biochem J. 1972 Feb;126(3):503–513. doi: 10.1042/bj1260503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Stahly D. P. Control of diaminopimelate decarboxylase by L-lysine during growth and sporulation of Bacilluscereus. J Bacteriol. 1971 May;106(2):551–560. doi: 10.1128/jb.106.2.551-560.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandgenett D. P., Stahly D. P. Diaminopimelate decarboxylase of sporulating bacteria. J Bacteriol. 1968 Dec;96(6):2099–2109. doi: 10.1128/jb.96.6.2099-2109.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoganson D. A., Irgens R. L., Doi R. H., Stahly D. P. Bacterial sporulation and regulation of dihydrodipicolinate synthase in ribonucleic acid polymerase mutants of Bacillus subtilis. J Bacteriol. 1975 Dec;124(3):1628–1629. doi: 10.1128/jb.124.3.1628-1629.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. C., Rush B. F. An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin Chem. 1966 May;12(5):299–307. [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahly D. P. Dihydrodipicolinic acid synthase of Bacillus licheniformis. Biochim Biophys Acta. 1969 Nov 4;191(2):439–451. doi: 10.1016/0005-2744(69)90263-0. [DOI] [PubMed] [Google Scholar]
- Vold B., Szulmajster J., Carbone A. Regulation of dihydrodipicolinate synthase and aspartate kinase in Bacillus subtilis. J Bacteriol. 1975 Mar;121(3):970–974. doi: 10.1128/jb.121.3.970-974.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters J. R., Stahly D. P. Modification of the valve of the French pressure cell. Appl Microbiol. 1968 Oct;16(10):1605–1605. doi: 10.1128/am.16.10.1605-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster F. H., Lechowich R. V. Partial purification and characterization of dihydrodipicolinic acid synthetase from sporulating Bacillus megaterium. J Bacteriol. 1970 Jan;101(1):118–126. doi: 10.1128/jb.101.1.118-126.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura F., Ikeda Y., Kimura K., Sasakawa T. Partial purification and some properties of pyruvate-aspartic semialdehyde condensing enzyme from sporulating Bacillus subtilis. J Biochem. 1974 Sep;76(3):611–621. doi: 10.1093/oxfordjournals.jbchem.a130605. [DOI] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]


