Abstract
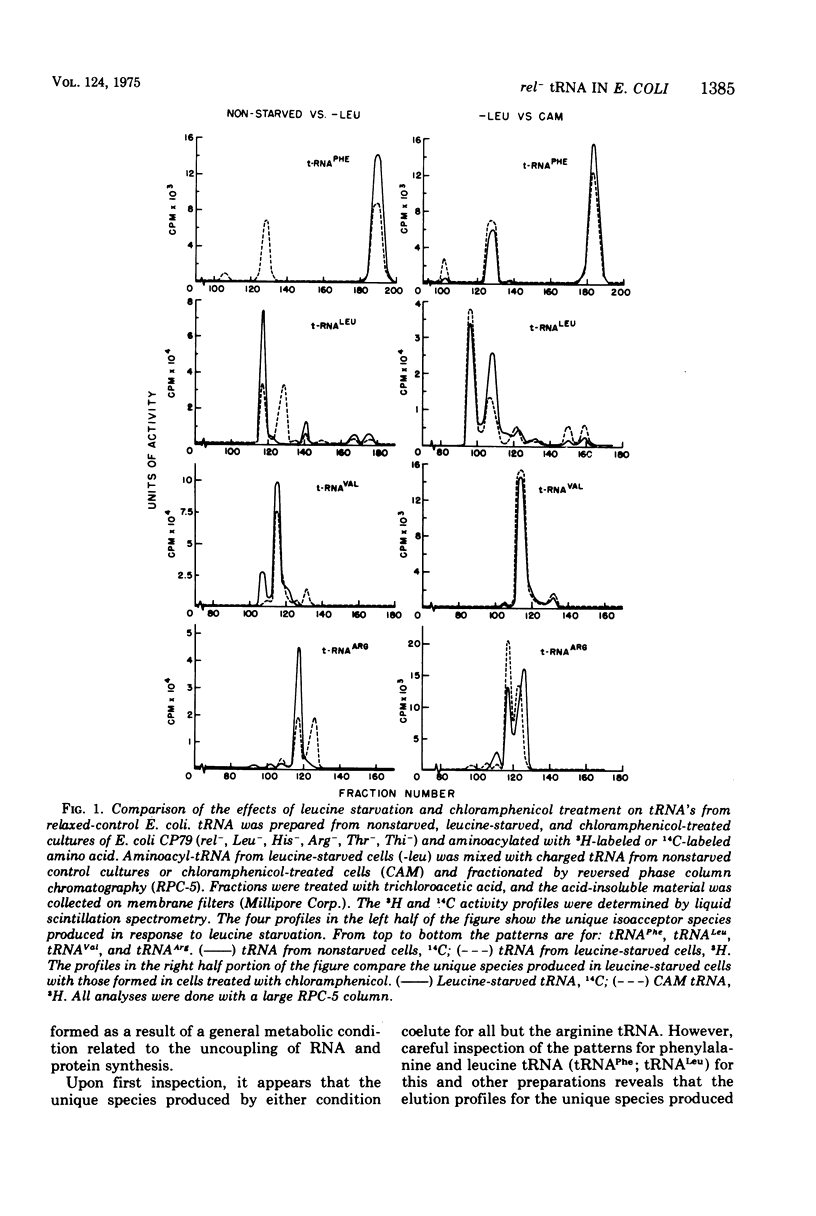
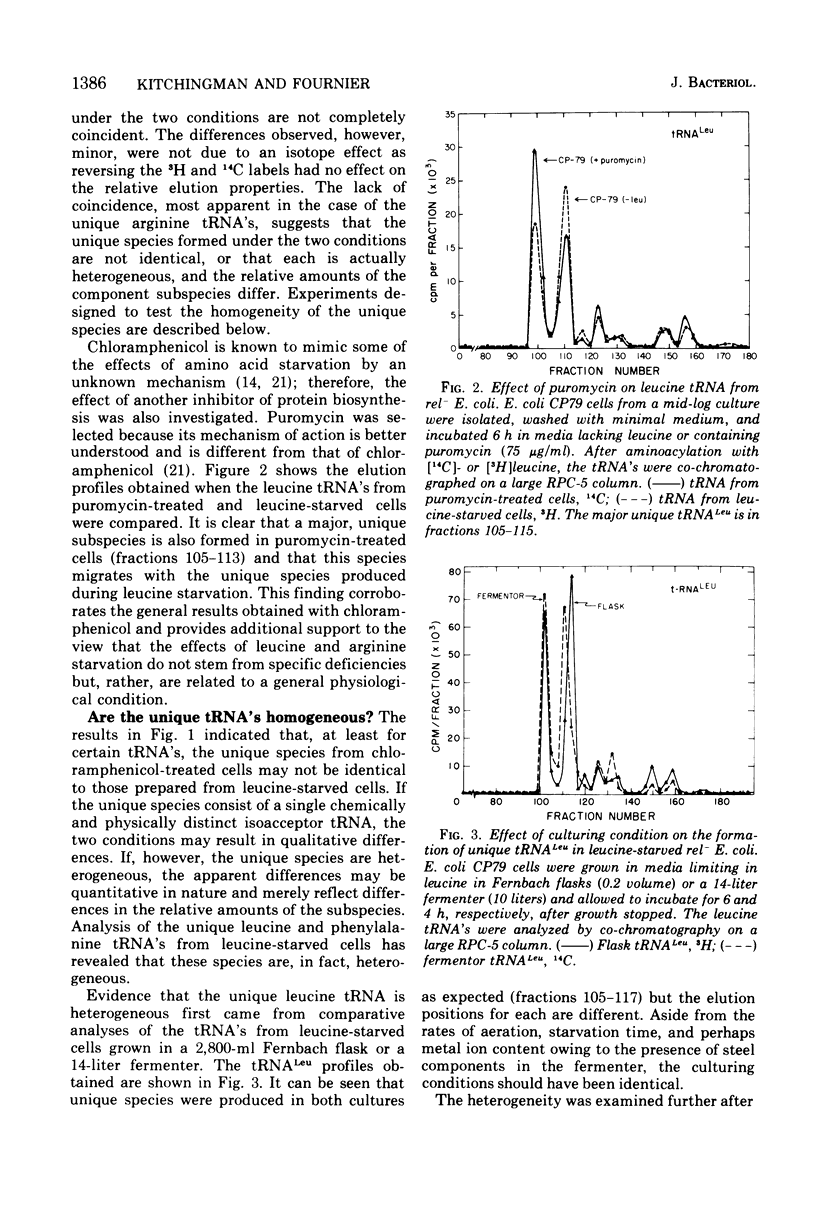
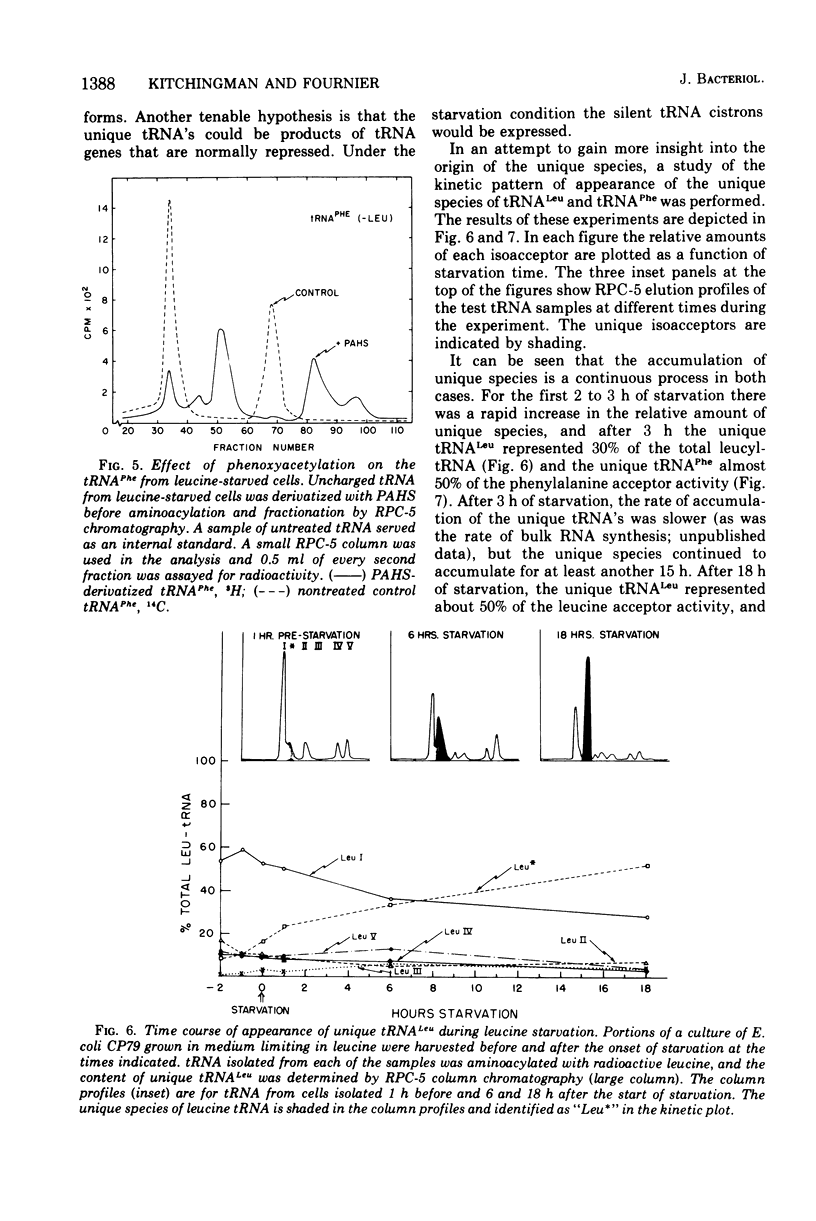
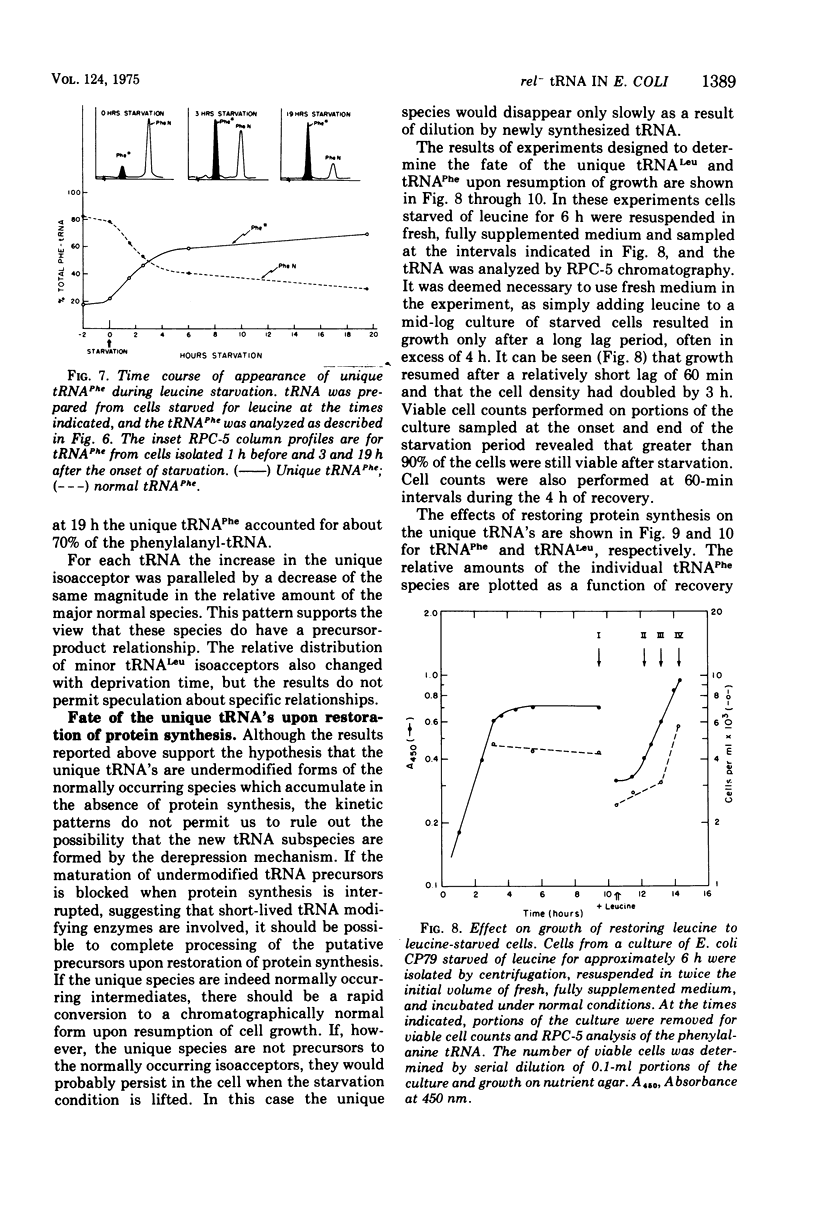
The unique leucine-, arginine-, valine-, and phenylalanine-specific transfer ribonucleic acids (tRNA's) produced in relaxed-control (rel-) Escherichia coli during leucine or arginine starvation are chromatographically similar to those produced by chloramphenicol treatment. The major unique rel- leucine-specific and phenylalanine-specific tRNA's are heterogeneous, accumulate with time of starvation, and can account for up to 70% of the respective amino acid acceptor activities. The changes which occur in the isoacceptor profiles for tRNALeu and tRNAPhe as a function of starvation time suggest that the unique species are undermodified precursors to the major isoacceptor species observed in nonstarved cells. Analyses of the isoacceptor patterns of tRNA from cells recovering from starvation suggest that the unique species of tRNALeu and tRNAPhe may not be normally occurring precursors. When leucine-starved cells were incubated in fresh, fully supplemented medium, the major unique tRNALeu and tRNAPhe appeared to be converted to normal species only slowly or not at all. The results are consistent with the view that some of the events in the post-transcriptional modification of tRNA may occur in an ordered sequence. An examination of the subcellular distribution of the unique leucine and phenylalanine tRNA's revealed that these species occur on the ribosome at about the same frequency as the major, normally occurring isoacceptor species. This result provides additional evidence of a precursor-product relationship for the unique and normal tRNA's and further indicates that there is no discrimination against the unique species by the ribosome.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chase R., Tener G. M., Gillam I. C. Changes in levels of amino acid acceptors in tRNA from Escherichia coli grown under various conditions. Arch Biochem Biophys. 1974 Jul;163(1):306–317. doi: 10.1016/0003-9861(74)90481-0. [DOI] [PubMed] [Google Scholar]
- Chheda G. B., Hong C. I., Piskorz C. F., Harmon G. A. Biosynthesis of N-(purin-6-ylcarbamoyl)-L-threonine riboside. Incorporation of L-threonine in vivo into modified nucleoside of transfer ribonucleic acid. Biochem J. 1972 Apr;127(3):515–519. doi: 10.1042/bj1270515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. J., Peterkofsky A. Formation of chromatographically unique species of transfer ribonucleic acid during amino acid starvation of relaxed-control Escherichia coli. J Bacteriol. 1975 May;122(2):538–548. doi: 10.1128/jb.122.2.538-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Goldberger R. F. Autogenous regulation of gene expression. Science. 1974 Mar 1;183(4127):810–816. doi: 10.1126/science.183.4127.810. [DOI] [PubMed] [Google Scholar]
- Harris C. L., Titchener E. B., Cline A. L. Sulfur-deficient transfer ribonucleic acid in a cysteine-requiring, "relaxed" mutant of Escherichia coli. J Bacteriol. 1969 Dec;100(3):1322–1327. doi: 10.1128/jb.100.3.1322-1327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Block R., Gilbert W., Weber K. MSI and MSII made on ribosome in idling step of protein synthesis. Nature. 1972 Aug 18;238(5364):381–384. doi: 10.1038/238381a0. [DOI] [PubMed] [Google Scholar]
- Huang P. C., Mann M. B. Comparative fingerprint and composition analysis of the three forms of 32P-labeled phenylalanine tRNA from chloramphenicol-treated Escherichia coli. Biochemistry. 1974 Nov 5;13(23):4704–4710. doi: 10.1021/bi00720a004. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Hedgcoth C. Levels of 5,6-dihydrouridine in relaxed and chloramphenicol transfer ribonucleic acid. Biochemistry. 1970 Jun 9;9(12):2513–2519. doi: 10.1021/bi00814a018. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- MANDEL L. R., BOREK E. Variability in the structure of ribonucleic acid. Biochem Biophys Res Commun. 1961 Jan 25;4:14–18. doi: 10.1016/0006-291x(61)90246-7. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Huang P. C. Behavior of chloramphenicol-induced phenylalanine transfer ribonucleic acid during recovery from chloramphenicol treatment in Escherichia coli. Biochemistry. 1973 Dec 18;12(26):5289–5294. doi: 10.1021/bi00750a011. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Huang P. C. New chromatographic form of phenylalanine transfer ribonucleic acid from Escherichia coli growing exponentially in a low-phosphate medium. J Bacteriol. 1974 Apr;118(1):209–212. doi: 10.1128/jb.118.1.209-212.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor J. B., Dickerman H. W., Peterkofsky A. Studies on methyl-deficient methionine transfer ribonucleic acid from Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3464–3473. [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Powers D. M., Peterkofsky A. Biosynthesis and specific labeling of N-(purin-6-ylcarbamoyl)threonine of Escherichia coli transfer RNA. Biochem Biophys Res Commun. 1972 Jan 31;46(2):831–838. doi: 10.1016/s0006-291x(72)80216-x. [DOI] [PubMed] [Google Scholar]
- Singer C. E., Smith G. R., Cortese R., Ames B. N. [Mutant tRNA His ineffective in repression and lacking two pseudouridine modifications]. Nat New Biol. 1972 Jul 19;238(81):72–74. doi: 10.1038/newbio238072a0. [DOI] [PubMed] [Google Scholar]
- Waters L. C. Altered chromatographic properties of tRNA from chloramphenicol-treated Escherichia coli. Biochem Biophys Res Commun. 1969 Oct 8;37(2):296–304. doi: 10.1016/0006-291x(69)90734-7. [DOI] [PubMed] [Google Scholar]
- Waters L. C., Shugart L., Yang W. K., Best A. N. Some physical and biological properties of 4-thiouridine- and dihydrouridine-deficient tRNA from chloramphenicol-treated Escherichia coli. Arch Biochem Biophys. 1973 Jun;156(2):780–793. doi: 10.1016/0003-9861(73)90332-9. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O. Differential in vivo aminoacylation and utilization of homologous species of E. coli transfer RNA. Cold Spring Harb Symp Quant Biol. 1966;31:595–599. doi: 10.1101/sqb.1966.031.01.077. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O., Stent G. S. Physiologically induced changes in the property of phenylalanine tRNA in Escherichia coli. J Mol Biol. 1968 Nov 28;38(1):25–40. doi: 10.1016/0022-2836(68)90126-5. [DOI] [PubMed] [Google Scholar]
- Williams L. S., Freundlich M. Role of valine transfer RNA in control of RNA synthesis in Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):515–517. doi: 10.1016/0005-2787(69)90064-1. [DOI] [PubMed] [Google Scholar]


