Abstract
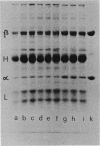
The cross-reaction of phenylalanyl-transfer ribonucleic acid (tRNA) ligases from different microorganisms with antibodies raised against the purified enzyme from Escherichia coli has been investigated. The results of immunotitration and immunodiffusion experiments and of the sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of immunoprecipitates revealed: (i) a high degree of immunochemical identity of this enzyme only within the family Enterobacteriaceae; (ii) intermediate-to-weak cross-reaction with the phenylalanyl-tRNA ligases from Pseudomonadaceae, Rhodopseudomonas spheroides, and Bacillus stearothermophilus; (iii) no detectable cross-reaction (with the methods employed) with the enzymes from several gram-positive organisms, Euglena gracilis, and several fungi. As revealed by immunochemical analysis, a merodiploid strain of E. coli carrying an episome (F148) that covers the aroD region of the E. coli chromosome possesses at least twice the amount of phenylalanyl-tRNA ligase in comparison with its haploid parent strain. This suggests that the cistrons for both the alpha and beta polypeptides of this enzyme are mapping in this area.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- Ebel J. P., Giegé R., Bonnet J., Kern D., Befort N., Bollack C., Fasiolo F., Gangloff J., Dirheimer G. Factors determining the specificity of the tRNA aminoacylation reaction. Non-absolute specificity of tRNA-aminoacyl-tRNA synthetase recognition and particular importance of the maximal velocity. Biochimie. 1973 May;55(5):547–557. doi: 10.1016/s0300-9084(73)80415-8. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., NEIDHARDT F. C. Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim Biophys Acta. 1961 Oct 14;53:96–110. doi: 10.1016/0006-3002(61)90797-1. [DOI] [PubMed] [Google Scholar]
- Fasiolo F., Befort N., Boulander Y., Ebel J. P. Purification et quelques propriétés de la phenylalanyl-tRNA synthetase de levure de boulangerie. Biochim Biophys Acta. 1970 Oct 15;217(2):305–318. [PubMed] [Google Scholar]
- Fayat G., Blanquet S., Dessen P., Batelier G., Waller J. P. The molecular weight and subunit composition of phenylalanyl-tRNA synthetase from Escherichia coli K-12. Biochimie. 1974;56(1):35–41. doi: 10.1016/s0300-9084(74)80353-6. [DOI] [PubMed] [Google Scholar]
- Hanke T., Bartmann P., Hennecke H., Kosakowski H. M., Jaenicke R., Holler E., Böck A. L-phenylalanyl-tRNA synthetase of Escherichia coli K-10. A reinvestigation of molecular weight and subunit structure. Eur J Biochem. 1974 Apr 16;43(3):601–607. doi: 10.1111/j.1432-1033.1974.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Hennecke H., Böck A. Altered alpha subunits in phenylalanyl-tRNA synthetases from p-fluorophenylalanine-resistant strains of Escherichis coli. Eur J Biochem. 1975 Jul 1;55(2):431–437. doi: 10.1111/j.1432-1033.1975.tb02179.x. [DOI] [PubMed] [Google Scholar]
- Kosakowski H. M., Böck A. Substrate complexes of phenylalanyl-tRNA synthetase from Escherichia coli. Eur J Biochem. 1971 Dec 22;24(1):190–200. doi: 10.1111/j.1432-1033.1971.tb19670.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Flashner M., Mckeever W. G., Neidhardt F. C. Metabolic regulation of the arginyl and valyl transfer ribonucleic acid synthetases in bacteria. J Biol Chem. 1974 Feb 25;249(4):1044–1053. [PubMed] [Google Scholar]
- Ritter P. O., Kull F. J., Jacobson K. B. Aminoacylation of Escherichia coli valine transfer ribonucleic acid by Neurospora crassa phenylalanyl transfer ribonucleic acid synthetase in tris(hydroxymethyl)aminomethane hydrochloric acid and potassium cacodylate buffers. Effect of salts, dimethyl sulfoxide, ethanol, and 2-mercaptoethanol. J Biol Chem. 1970 Apr 25;245(8):2114–2120. [PubMed] [Google Scholar]
- Schmidt J., Wang R., Stanfield S., Reid B. R. Yeast phenylalanyl transfer ribonucleic acid synthetase. Purification, molecular weight, and subunit structure. Biochemistry. 1971 Aug 17;10(17):3264–3268. doi: 10.1021/bi00793a016. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tscherne J. S., Lanks K. W., Salim P. D., Grunberger D., Cantor C. R., Weinstein I. B. Studies on rat liver phenylalanyl transfer ribonucleic acid synthetase. II. Further purification, substrate specificity, and effects of substrates on heat inactivation. J Biol Chem. 1973 Jun 10;248(11):4052–4059. [PubMed] [Google Scholar]