Abstract
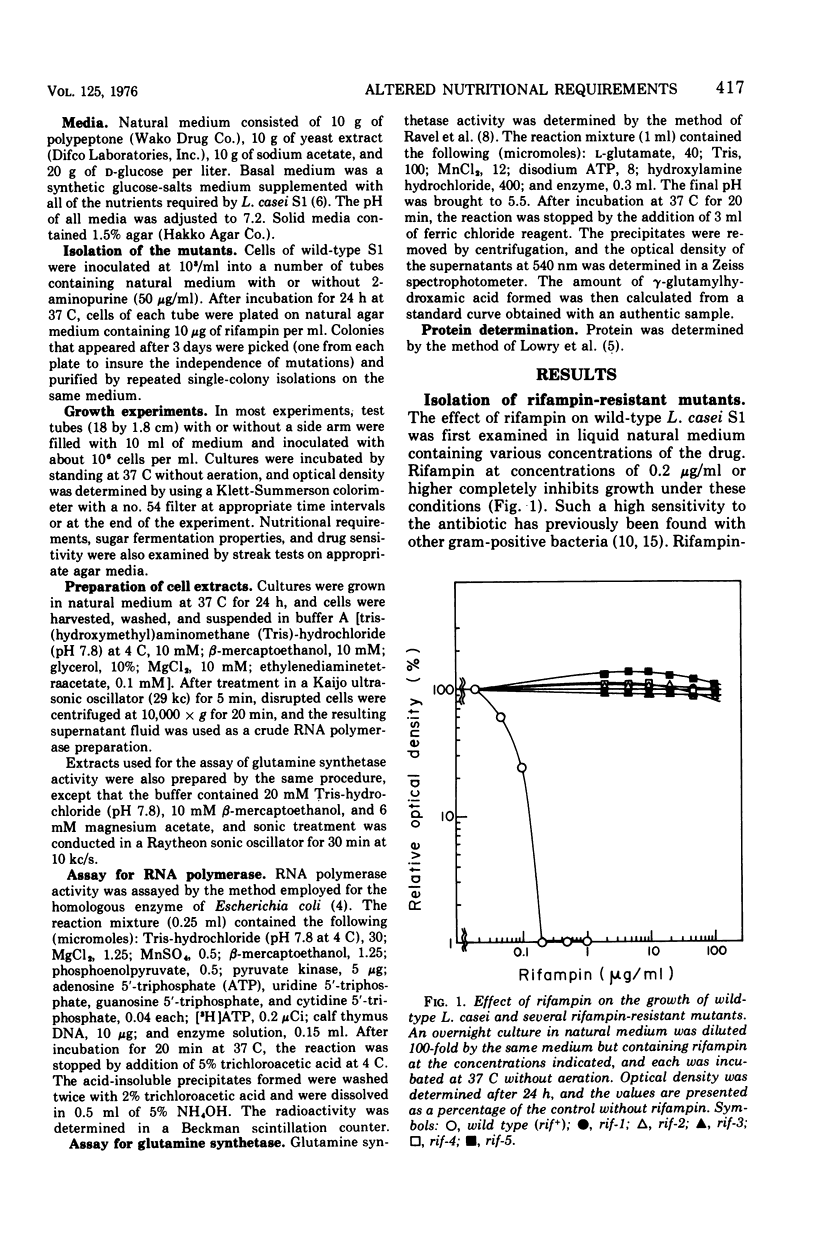
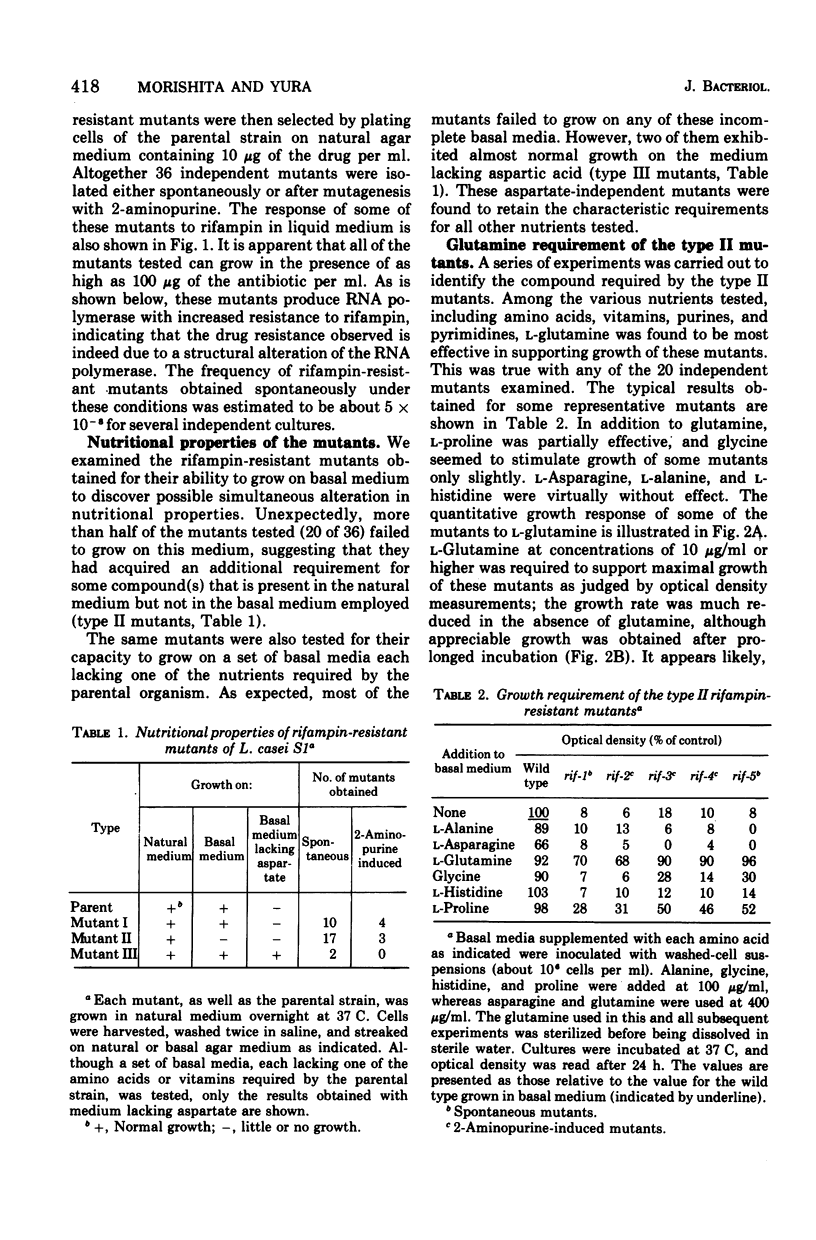
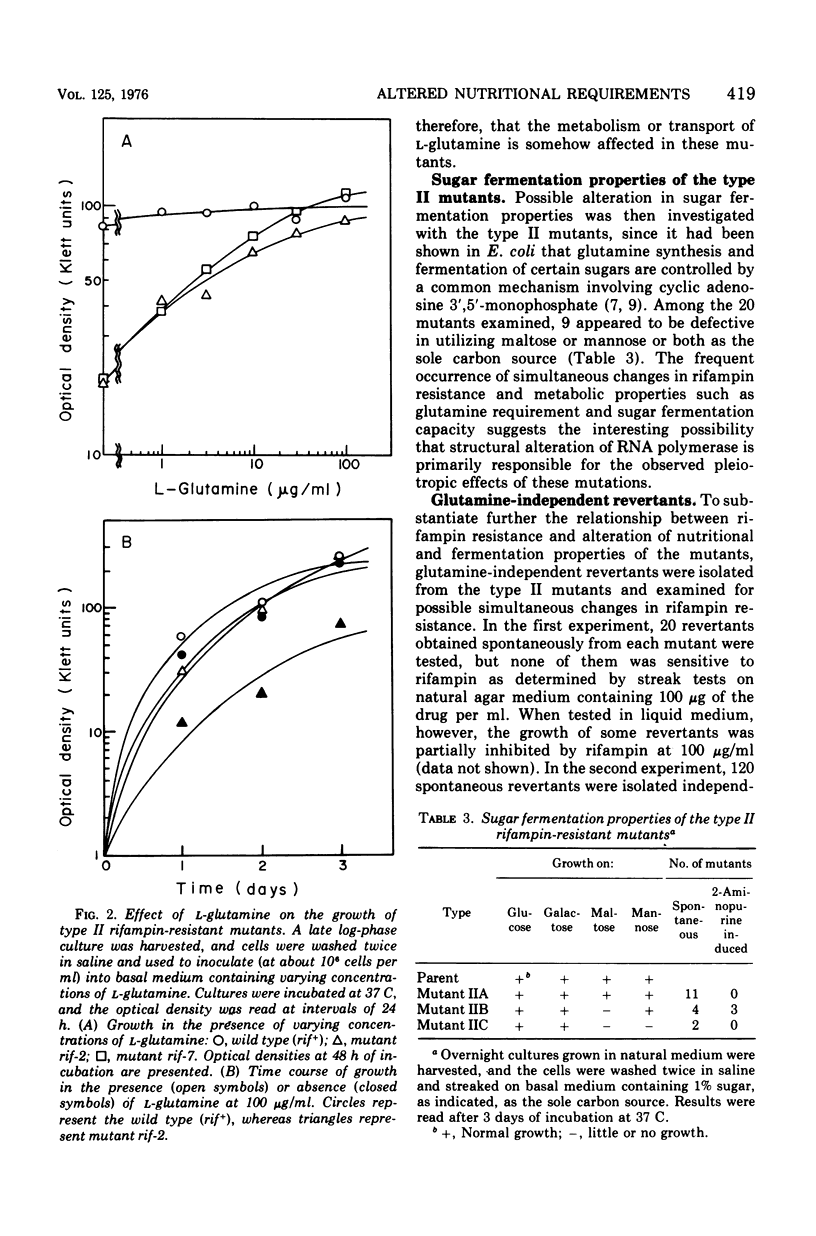
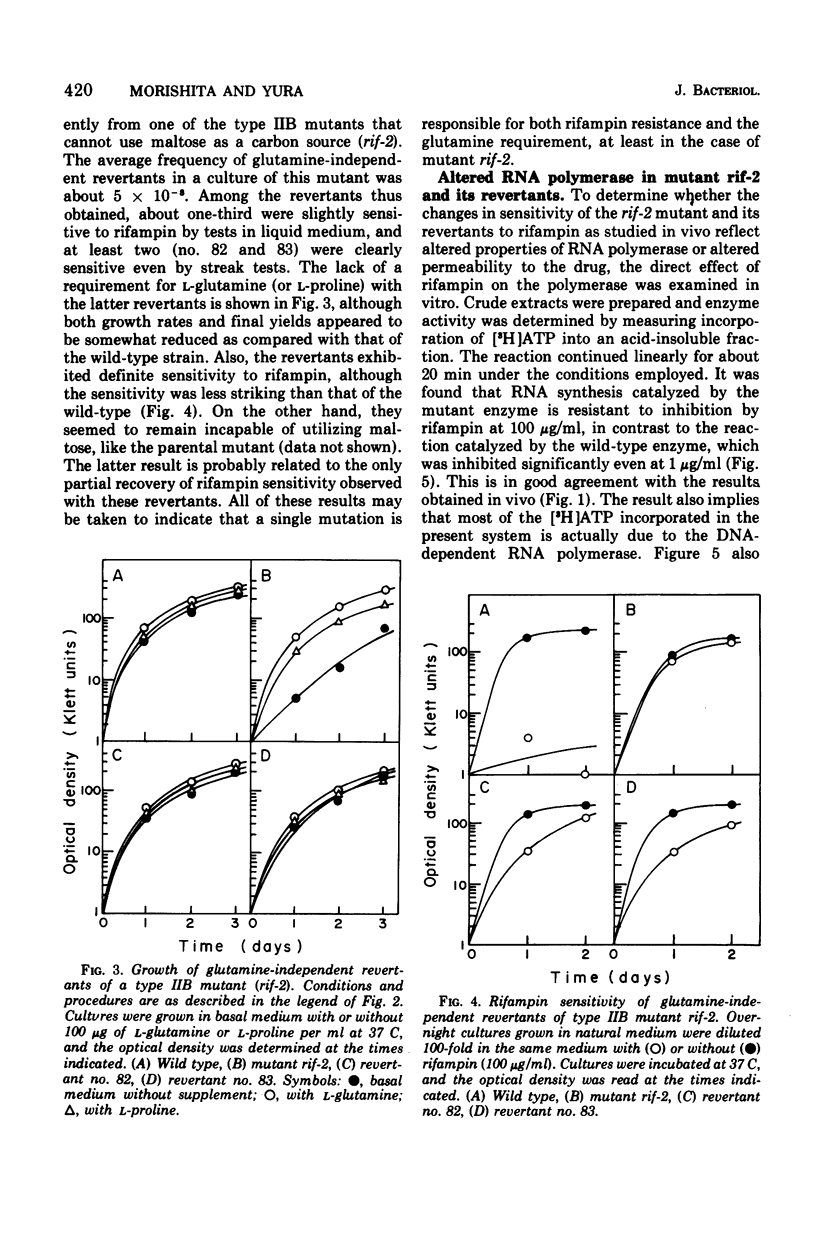
Rifampin-resistant mutants were isolated from Lactobacillus casei S1 and examined for possible simultaneous alteration in nutritional properties. Among the 36 mutants obtained either spontaneously or after mutagenesis with 2-aminopurine, 22 were found to be altered with respect to the specific growth requirements. The majority (20 of 22) of the latter mutants were shown to require L-glutamine in addition to the nutrients required by the parental strain for maximal growth, whereas the remaining mutants had apparently lost the requirement for L-aspartate. Further studies with one of the glutamine-requiring mutants revealed that the rifampin resistance of this strain is due to the resistance of ribonucleic acid polymerase itself and that a single mutation is responsible for both rifampin resistance and the glutamine requirement. These results strongly indicate that a structural alteration of the ribonucleic acid polymerase caused by the rifampin resistance mutation somehow affected glutamine metabolism, possibly through change in selective transcription of the genes involved.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A., Yura T. RNA polymerase mutants of Escherichia coli. Streptolydigin resistance and its relation to rifampicin resistance. Mol Gen Genet. 1973 Mar 1;121(2):181–196. doi: 10.1007/BF00277531. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morishita T., Fukada T., Shirota M., Yura T. Genetic basis of nutritional requirements in Lactobacillus casei. J Bacteriol. 1974 Dec;120(3):1078–1084. doi: 10.1128/jb.120.3.1078-1084.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M., Humphreys J. S., Shive W. Control of glutamine synthesis in Lactobacillus arabinosus. Arch Biochem Biophys. 1965 Sep;111(3):720–726. doi: 10.1016/0003-9861(65)90255-9. [DOI] [PubMed] [Google Scholar]
- Rickenberg H. V. Cyclic AMP in prokaryotes. Annu Rev Microbiol. 1974;28(0):353–369. doi: 10.1146/annurev.mi.28.100174.002033. [DOI] [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- Sethi V. S. Structure and function of DNA-dependent RNA-polymerase. Prog Biophys Mol Biol. 1971;23:67–101. doi: 10.1016/0079-6107(71)90017-4. [DOI] [PubMed] [Google Scholar]
- Snyder L. R. An RNA polymerase mutant of Escherichia coli defective in the T4 viral transcription program. Virology. 1972 Nov;50(2):396–403. doi: 10.1016/0042-6822(72)90391-1. [DOI] [PubMed] [Google Scholar]
- Sonenshein A. L., Losick R. RNA polymerase mutants blocked in sporulation. Nature. 1970 Aug 29;227(5261):906–909. doi: 10.1038/227906a0. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


