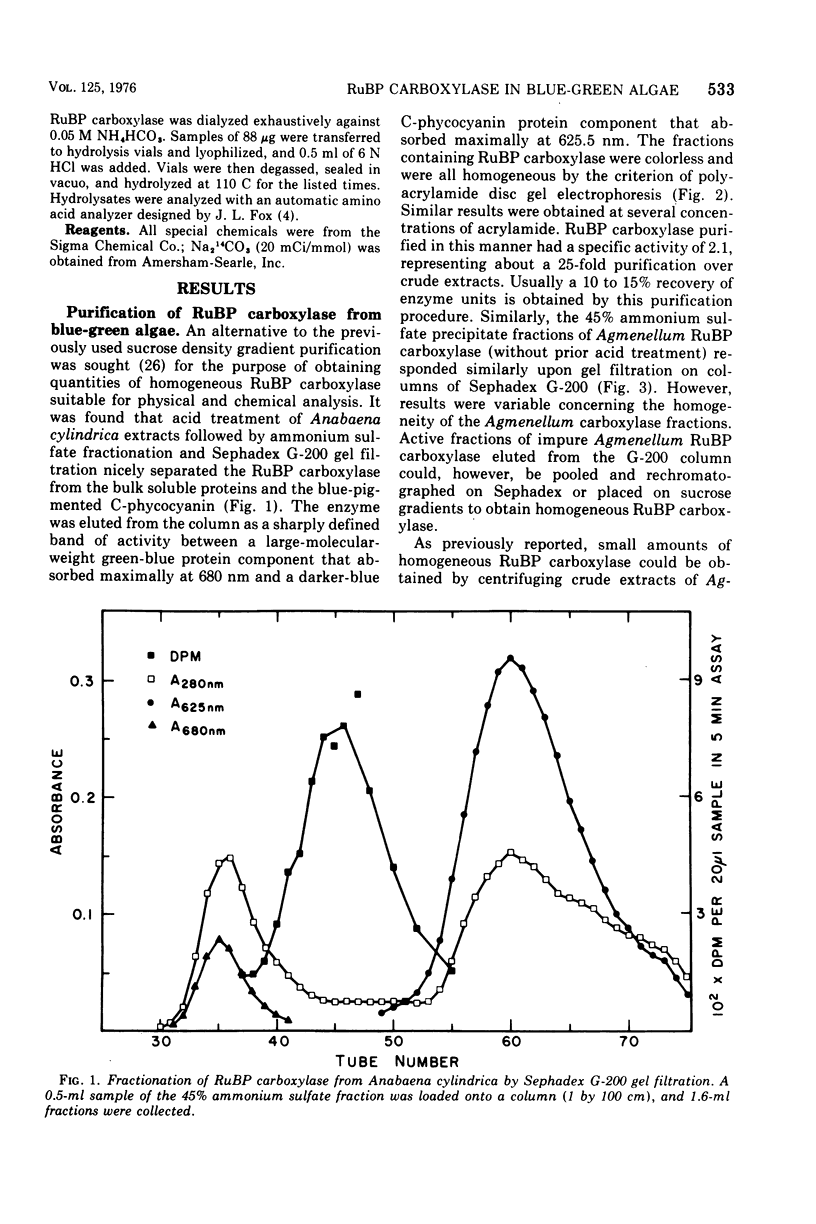
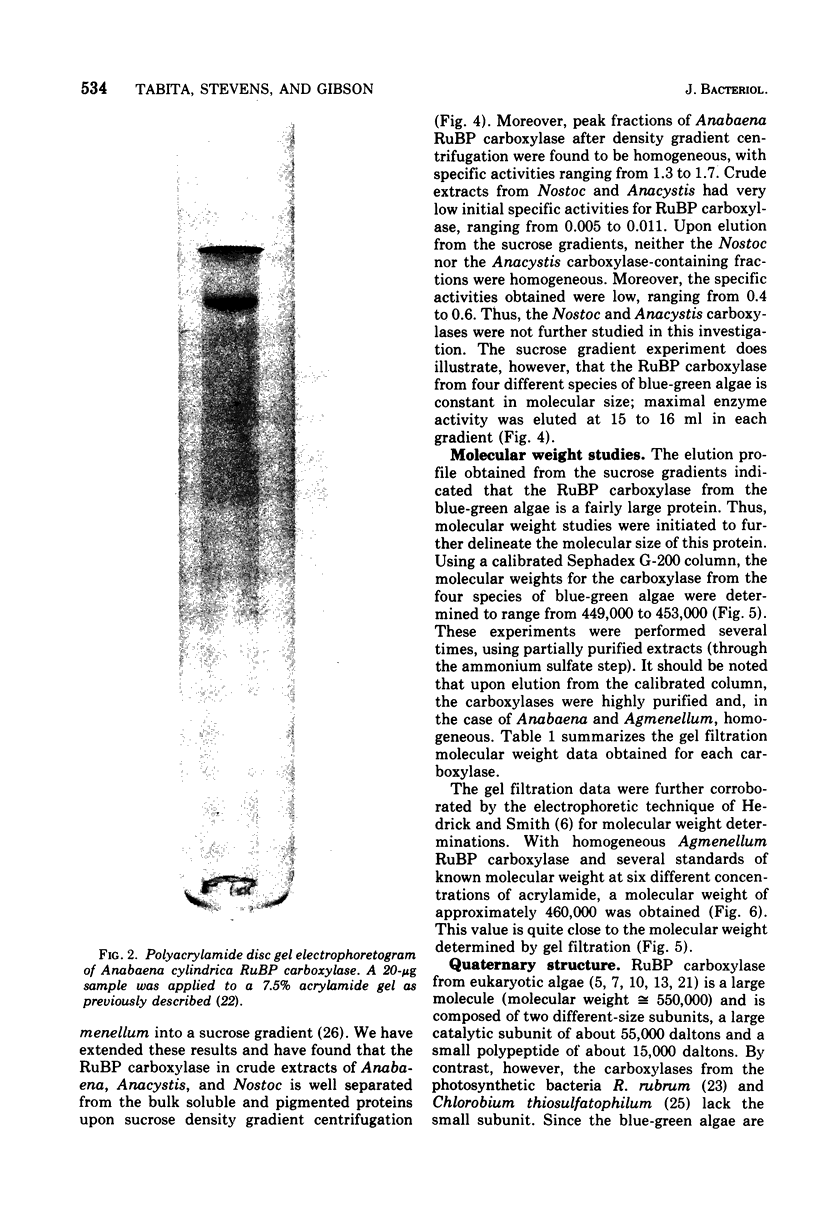
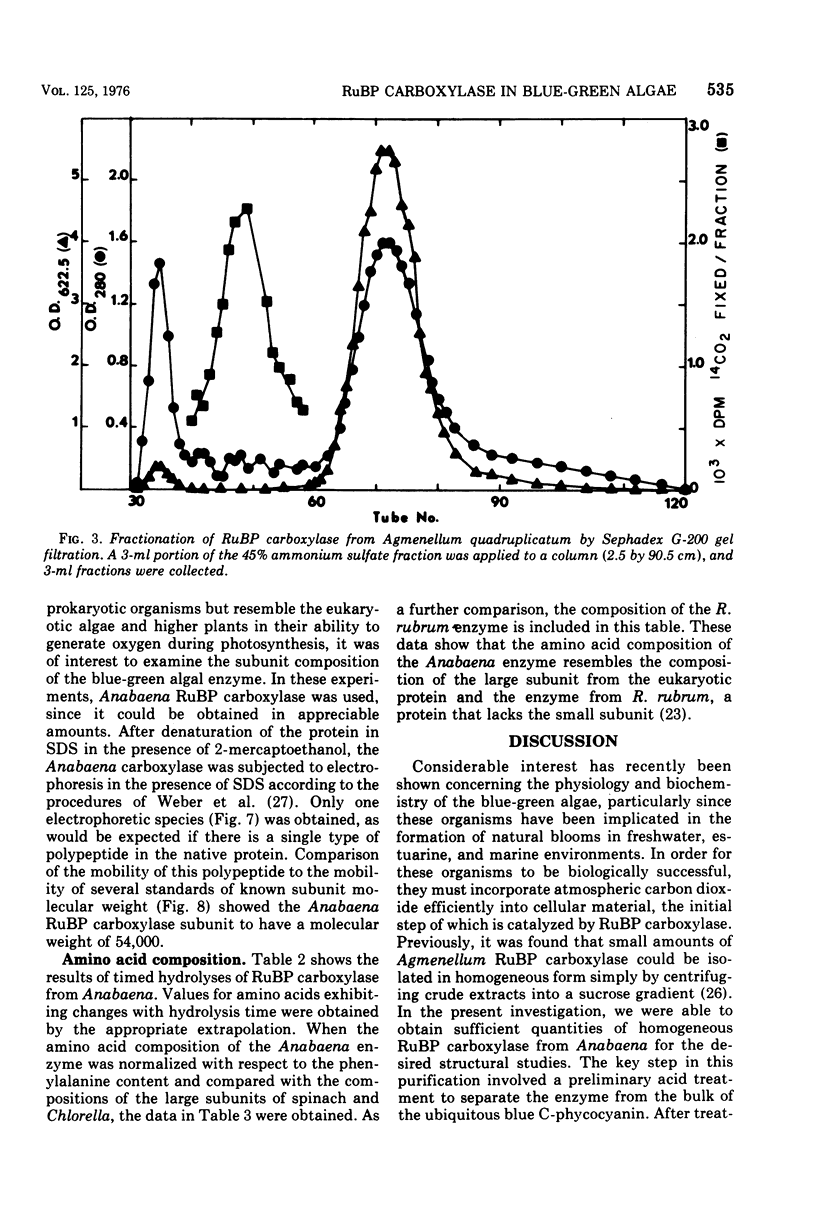
Abstract
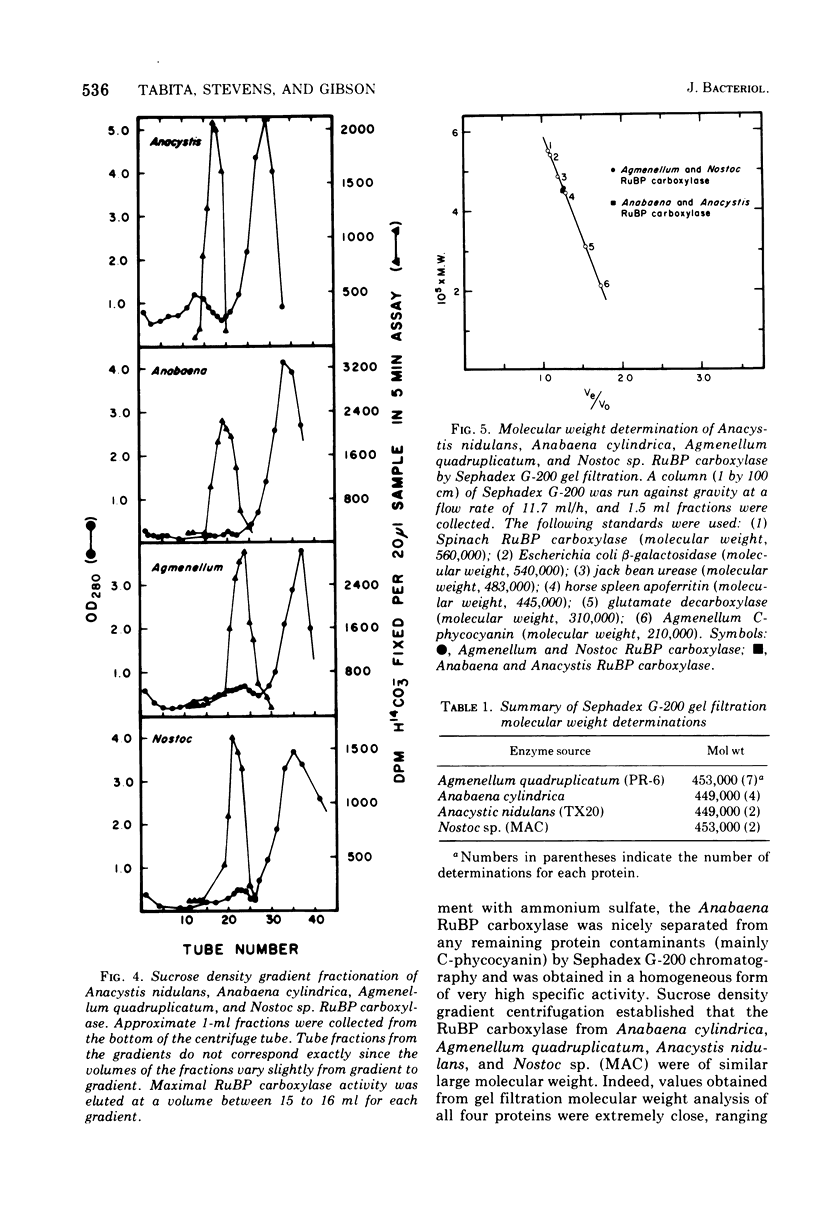
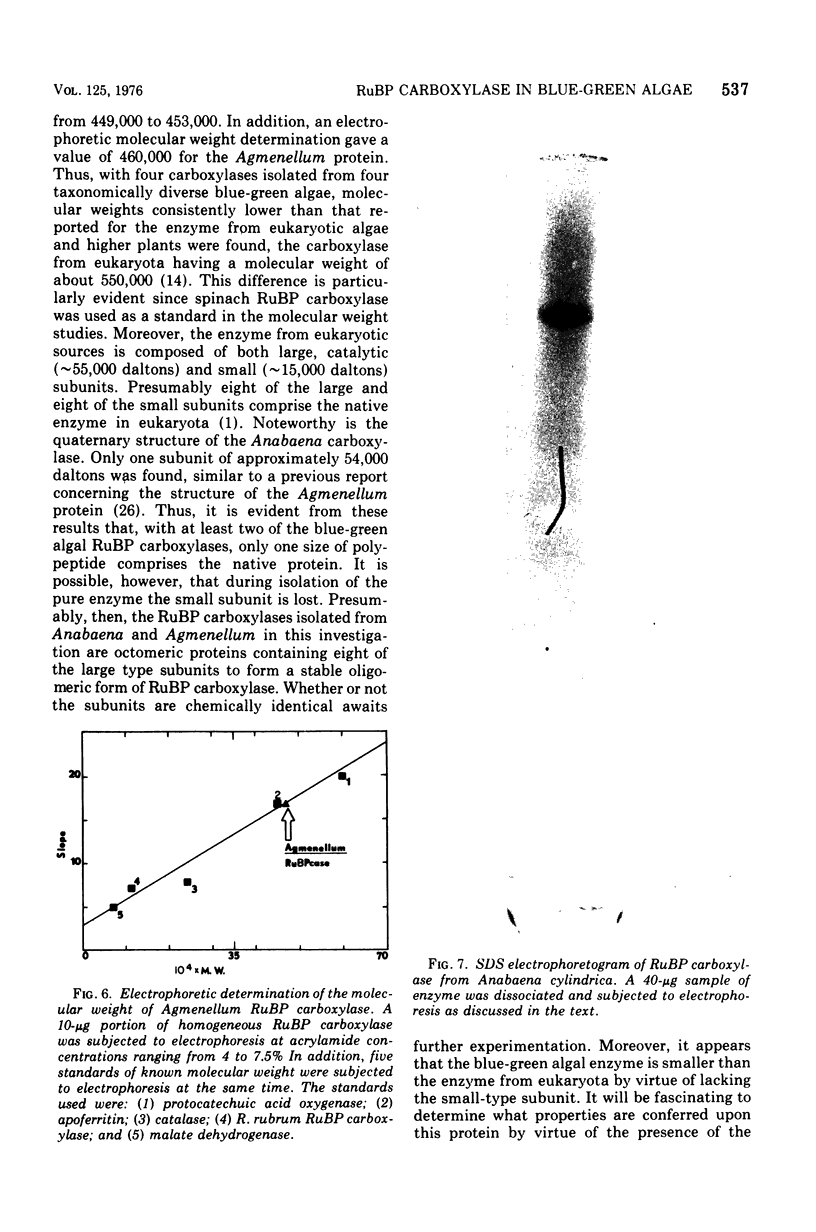
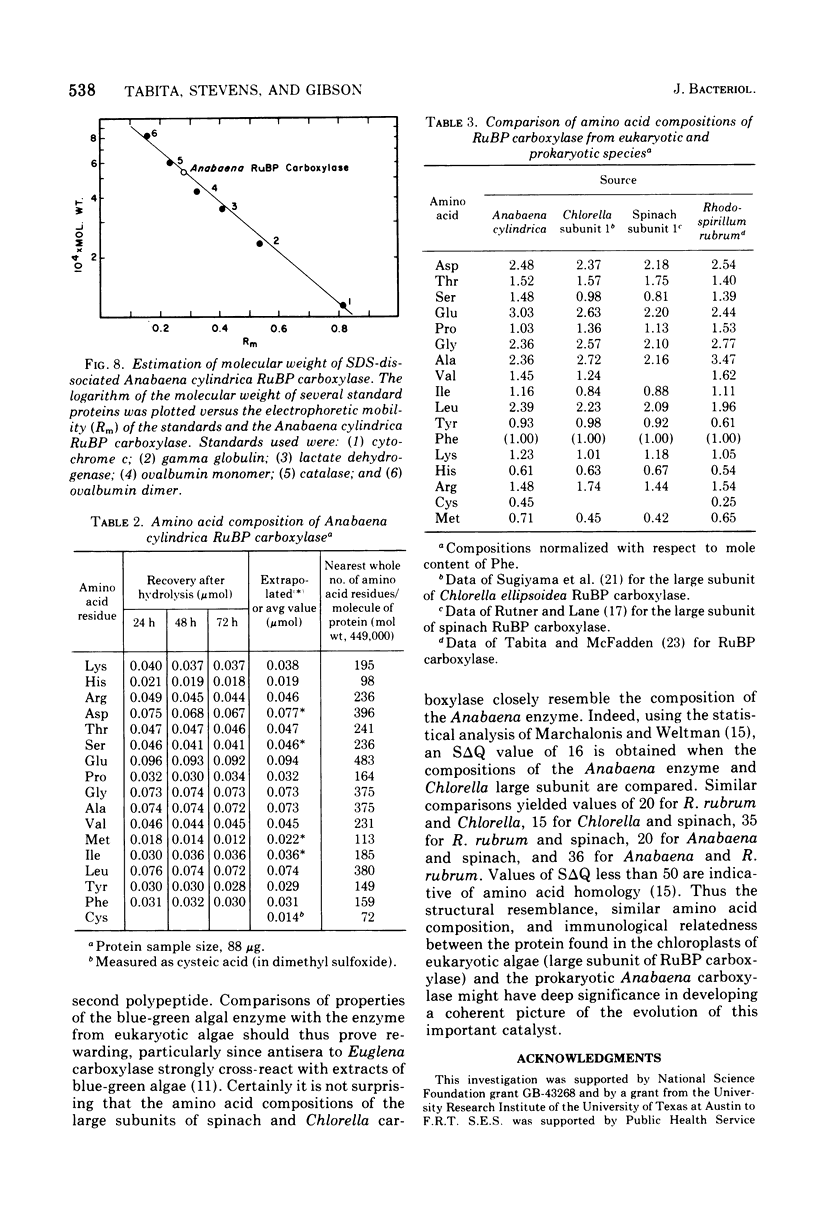
D-Ribulose 1,5-bisphosphate carboxylase was purified from the blue-green alga Anabaena cylindrica (Lemm) by procedures involving acid precipitation, ammonium sulfate fractionation, and Sephadex G-200 gel filtration. The enzyme was homogeneous by the criterion of polyacrylamide disc gel electrophoresis and was a multimer of a single-size polypeptide chain of 54,000 daltons. The carboxylases from four species of blue-green algae (Anabaena, Nostoc strain MAC, Agmenellum quadruplicatum strain PR-6, and Anacystis nidulans strain TX20) were closely similar in molecular size, since enzyme activity was eluted at the same volume after sucrose gradient centrifugation. Further analysis by gel filtration indicated that the four blue-green algal carboxylases were nearly identical in molecular weight, ranging from 449 to 453,000. The amino acid composition of the Anabaena carboxylase was determined and was found to resemble closely the composition of the large subunit from eukaryotic photosynthetic organisms.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Eisenberg D., Eiserling F. A., Weissman L. The structure of form I crystals of D-ribulose-1,5-diphosphate carboxylase. J Mol Biol. 1975 Feb 5;91(4):391–399. doi: 10.1016/0022-2836(75)90267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnall D. W., Klotz I. M. Subunit constitution of proteins: a table. Arch Biochem Biophys. 1975 Feb;166(2):651–682. doi: 10.1016/0003-9861(75)90432-4. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fox J. L., Smith S. S., Brown J. R. Amino acid sequences of Clostridium pasteurianum flavodoxin. Z Naturforsch B. 1972 Sep;27(9):1096–1100. doi: 10.1515/znb-1972-0932. [DOI] [PubMed] [Google Scholar]
- Givan A. L., Criddle R. S. Ribulosediphosphate carboxylase from Chlamydomonas reinhardi: purification, properties and its mode of synthesis in the cell. Arch Biochem Biophys. 1972 Mar;149(1):153–163. doi: 10.1016/0003-9861(72)90309-8. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Jansz E. R., Maclean F. I. CO 2 fixation by the blue-green alga Anacystis nidulans. Can J Microbiol. 1973 Apr;19(4):497–504. doi: 10.1139/m73-080. [DOI] [PubMed] [Google Scholar]
- Kato M., Lee W. I., Eichinger B. E., Schurr J. M. Molecular weight and shape of the phycocyanin hexamer. Biopolymers. 1974 Nov;13(11):2293–2304. doi: 10.1002/bip.1974.360131111. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Brown R. H. Purification and Some Properties of Chlorella fusca Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1975 Feb;55(2):360–364. doi: 10.1104/pp.55.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Lord J. M., Rowe A., Dilks S. Composition, quaternary structure, and catalytic properties of D-ribulose-1, 5-bisphosphate carboxylase from Euglena gracilis. Eur J Biochem. 1975 May;54(1):195–206. doi: 10.1111/j.1432-1033.1975.tb04129.x. [DOI] [PubMed] [Google Scholar]
- McFadden B. A., Tabita F. R. D-ribulose-1, 5-diphosphate carboxylase and the evolution of autotrophy. Biosystems. 1974 Oct;6(2):93–112. doi: 10.1016/0303-2647(74)90002-1. [DOI] [PubMed] [Google Scholar]
- Myers D. M. Procedure for drying leptospiral antibody on sand and sugar for serological studies in leptospirosis. Appl Microbiol. 1973 Mar;25(3):427–430. doi: 10.1128/am.25.3.427-430.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelroy R. A., Bassham J. A. Photosynthetic and dark carbon metabolism in unicellular blue-green algae. Arch Mikrobiol. 1972;86(1):25–38. doi: 10.1007/BF00412397. [DOI] [PubMed] [Google Scholar]
- Rutner A. C., Lane M. D. Nonidentical subunits of ribulose diphosphate carboxylase. Biochem Biophys Res Commun. 1967 Aug 23;28(4):531–537. doi: 10.1016/0006-291x(67)90346-4. [DOI] [PubMed] [Google Scholar]
- Siegel M. I., Lane M. D. Chemical and enzymatic evidence for the participation of a 2-carboxy-3-ketoribitol-1,5-diphosphate intermediate in the carboxylation of ribulose 1,5-diphosphate. J Biol Chem. 1973 Aug 10;248(15):5486–5498. [PubMed] [Google Scholar]
- Sjödin B., Vestermark A. The enzymatic formation of a compound with the expected properties of carboxylated ribulose 1,5-diphosphate. Biochim Biophys Acta. 1973 Jan 24;297(1):165–173. doi: 10.1016/0304-4165(73)90060-3. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Ito T., Akazawa T. Subunit structure of ribulose 1,5-diphosphate carboxylase from Chlorella ellipsoidea. Biochemistry. 1971 Aug 31;10(18):3406–3411. doi: 10.1021/bi00794a014. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. I. Levels, purification, and effects of metallic ions. J Biol Chem. 1974 Jun 10;249(11):3453–3458. [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. D-ribulose 1,5-diphosphate carboxylase from Rhodospirillum rubrum. II. Quaternary structure, composition, catalytic, and immunological properties. J Biol Chem. 1974 Jun 10;249(11):3459–3464. [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A. One-step isolation of microbial ribulose-1,5-diphosphate carboxylase. Arch Microbiol. 1974;99(3):231–240. doi: 10.1007/BF00696237. [DOI] [PubMed] [Google Scholar]
- Tabita F. R., McFadden B. A., Pfennig N. D-ribulose-1,5-bisphosphate carboxylase in Chlorobium thiosulfatophilum Tassajara. Biochim Biophys Acta. 1974 Mar 21;341(1):187–194. doi: 10.1016/0005-2744(74)90079-5. [DOI] [PubMed] [Google Scholar]
- Tabita R. F., Stevens S. E., Jr, Quijano R. D-ribulose 1, 5-diphosphate carboxylase from blue-green algae. Biochem Biophys Res Commun. 1974 Nov 6;61(1):45–52. doi: 10.1016/0006-291x(74)90531-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]