Abstract
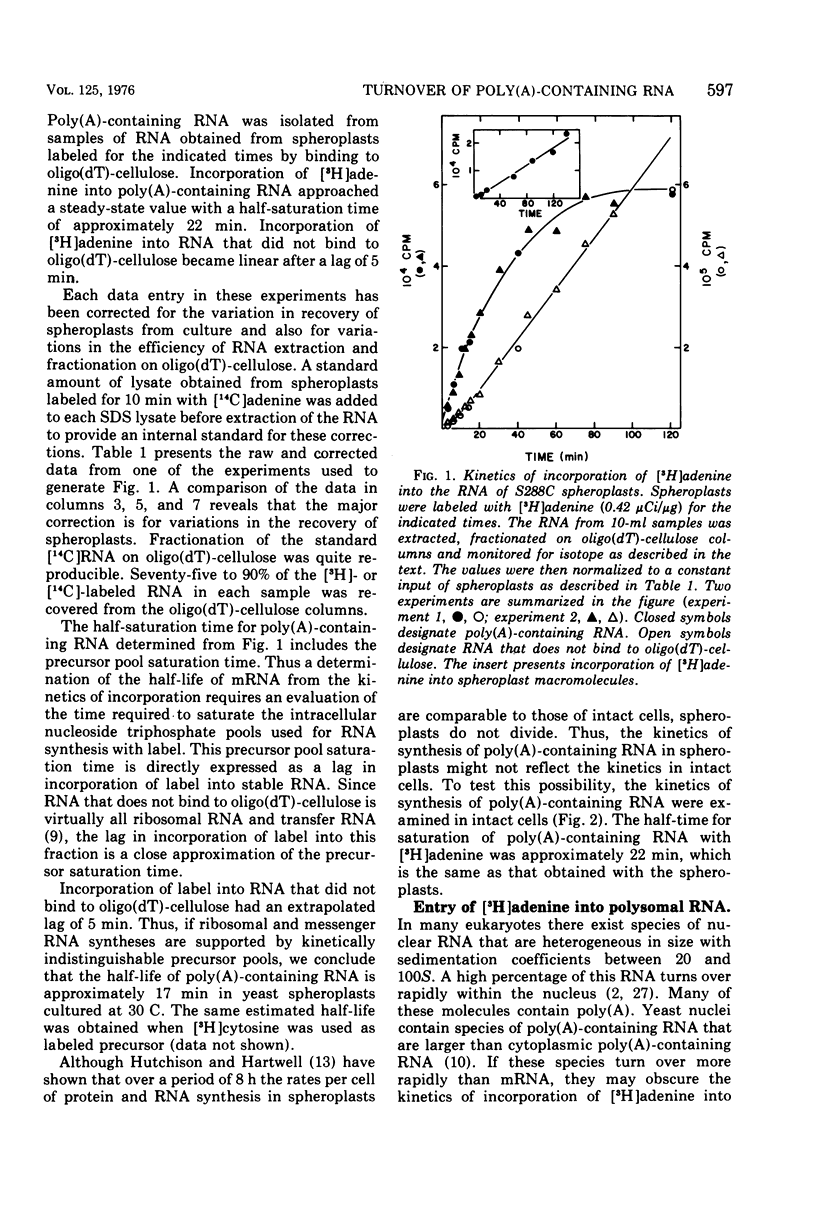
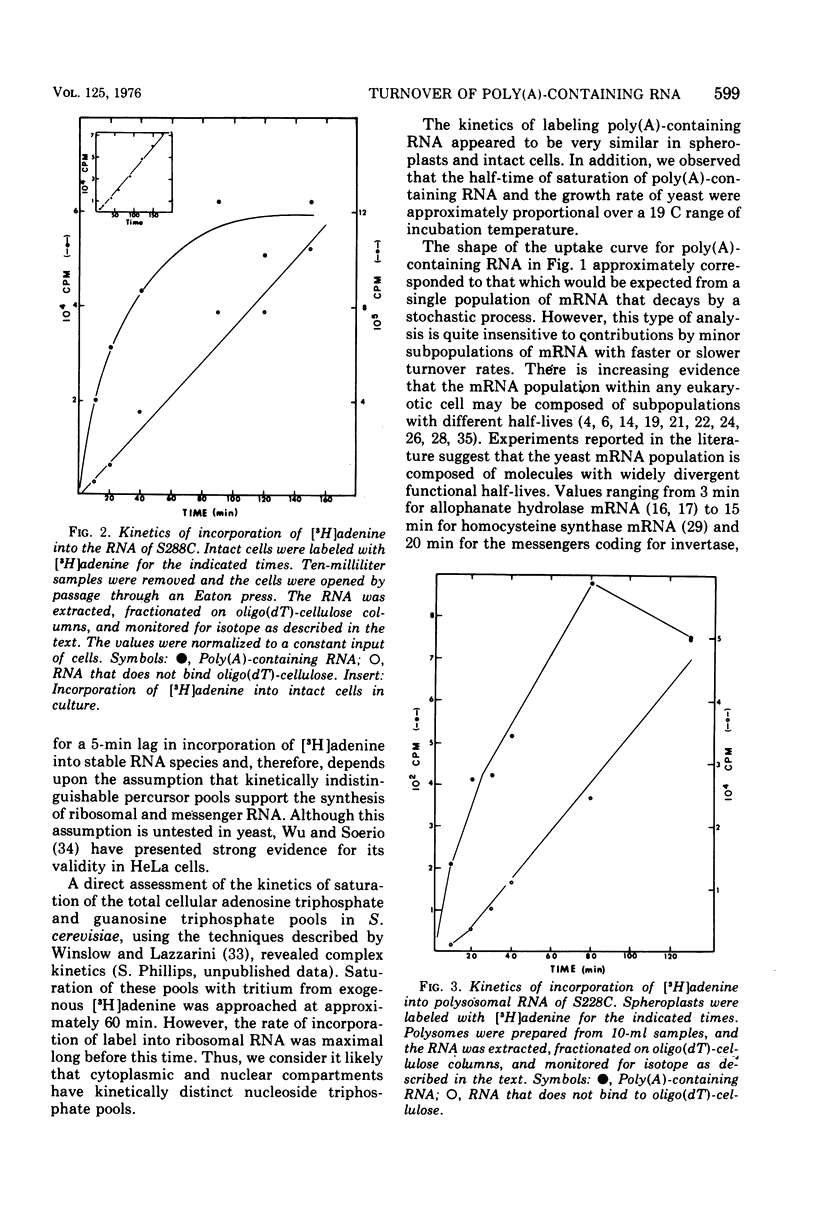
We examined the kinetics of incorporation of [3H]adenine into polyadenylate-containing ribonucleic acid [poly(A)-containing RNA] in yeast. The total poly(A)-containing RNA from spheroplasts and intact cells and the polysomal poly(A)-containing RNA exhibited similar incorporation kinetics. At 30 C half-saturation of the pool of poly(A)-containing RNA with label occurred in approximately 22 min. Since precursor pools appeared to require 5 min to saturate with label, we conclude that at 30 C messenger RNA molecules in yeast decay with an average half-life of 17 min.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Induction of galactokinase in Saccharomyces cerevisiae: kinetics of induction and glucose effects. J Bacteriol. 1972 Aug;111(2):308–315. doi: 10.1128/jb.111.2.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- Cannon M., Davies J. E., Jimenez A. Inhibition by lomofungin of nucleic acid and protein synthesis in Saccharomyces cerevisiae. FEBS Lett. 1973 Jun 1;32(2):277–280. doi: 10.1016/0014-5793(73)80852-x. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Sheinin R. Selective measurement of the synthesis and metabolic stability of messenger RNA in 3T3 mouse cells. Biochim Biophys Acta. 1970 Apr 15;204(2):449–461. doi: 10.1016/0005-2787(70)90165-6. [DOI] [PubMed] [Google Scholar]
- Fantoni A., De la Chapelle A., Rifkind R. A., Marks P. A. Erythroid cell-development in fetal mice: synthetic capacity for different proteins. J Mol Biol. 1968 Apr 14;33(1):79–91. doi: 10.1016/0022-2836(68)90282-9. [DOI] [PubMed] [Google Scholar]
- Greenberg J. R. High stability of messenger RNA in growing cultured cells. Nature. 1972 Nov 10;240(5376):102–104. doi: 10.1038/240102a0. [DOI] [PubMed] [Google Scholar]
- Groner B., Hynes N., Phillips S. Length heterogeneity in the poly (adenylic acid) region of yeast messenger ribonucleic acid. Biochemistry. 1974 Dec 17;13(26):5378–5383. doi: 10.1021/bi00723a020. [DOI] [PubMed] [Google Scholar]
- Groner B., Phillips S. L. Polyadenylate metabolism in the nuclei and cytoplasm of Saccharomyces cerevisiae. J Biol Chem. 1975 Jul 25;250(14):5640–5646. [PubMed] [Google Scholar]
- Hartwell L. H., Hutchison H. T., Holland T. M., McLaughlin C. S. The effect of cycloheximide upon polyribosome stability in two yeast mutants defective respectively in the initiation of polypeptide chains and in messenger RNA synthesis. Mol Gen Genet. 1970;106(4):347–361. doi: 10.1007/BF00324052. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H. Macromolecule synthesis in yeast spheroplasts. J Bacteriol. 1967 Nov;94(5):1697–1705. doi: 10.1128/jb.94.5.1697-1705.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C. The cocoonase zymogen cells of silk moths: a model of terminal cell differentiation for specific protein synthesis. Curr Top Dev Biol. 1972;7:125–191. doi: 10.1016/s0070-2153(08)60071-x. [DOI] [PubMed] [Google Scholar]
- Klo S. C., Cano F. R., Lampen J. O. Lomofungin, an inhibitor of ribonucleic acid synthesis in yeast protoplasts: its effect on enzyme formation. Antimicrob Agents Chemother. 1973 Jun;3(6):716–722. doi: 10.1128/aac.3.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Effects of inducer addition and removal upon the level of allophanate hydrolase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1100–1104. doi: 10.1016/s0006-291x(73)80008-7. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Warner J. R., Edmonds M., Nakazato H., Vaughan M. H. Polyadenylic acid sequences in yeast messenger ribonucleic acid. J Biol Chem. 1973 Feb 25;248(4):1466–1471. [PubMed] [Google Scholar]
- Palmiter R. D. Regulation of protein synthesis in chick oviduct. I. Independent regulation of ovalbumin, conalbumin, ovomucoid, and lysozyme induction. J Biol Chem. 1972 Oct 25;247(20):6450–6461. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Regier J. C., Kafatos F. C. Microtechnique for determining the specific activity of radioactive intracellular leucine and applications to in vivo studies of protein synthesis. J Biol Chem. 1971 Nov;246(21):6480–6488. [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz G. A. The stability of messenger RNA containing polyadenylic acid sequences in rabbit blastocysts. Exp Cell Res. 1974 May;86(1):190–193. doi: 10.1016/0014-4827(74)90671-5. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Messenger RNA in HeLa cells: kinetics of formation and decay. J Mol Biol. 1973 Aug 5;78(2):321–334. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A., Hui H., Penman S. Two very different components of messenger RNA in an insect cell line. Cell. 1975 Feb;4(2):131–137. doi: 10.1016/0092-8674(75)90119-1. [DOI] [PubMed] [Google Scholar]
- Surdin-Kerjan Y., de Robichon-Szulmajster H. Existence of two levels of repression in the biosynthesis of methionine in Saccharomyces cerevisiae: effect of lomofungin on enzyme synthesis. J Bacteriol. 1975 May;122(2):367–374. doi: 10.1128/jb.122.2.367-374.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udem S. A., Warner J. R. The cytoplasmic maturation of a ribosomal precursor ribonucleic acid in yeast. J Biol Chem. 1973 Feb 25;248(4):1412–1416. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Winslow R. M., Lazzarini R. A. The rates of synthesis and chain elongation of ribonucleic acid in Escherichia coli. J Biol Chem. 1969 Mar 10;244(5):1128–1136. [PubMed] [Google Scholar]
- Wu R. S., Soeiro R. Turnover of nuclear RNA in HeLa cells: evidence for a single ribonucleotide pool. J Mol Biol. 1971 Jun 14;58(2):481–487. doi: 10.1016/0022-2836(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Yund M. A., Kafatos F. C., Regier J. C. Posttranscriptional regulation of specific protein synthesis in the silkmoth wing, as revealed by actinomycin D. Dev Biol. 1973 Aug;33(2):362–379. doi: 10.1016/0012-1606(73)90143-7. [DOI] [PubMed] [Google Scholar]


