Abstract
In this minireview, we briefly revisit the Drosophila Notch and epidermal growth factor receptor pathways, and relate to the relationship between them. We then mainly focus on the involvement of Groucho (Gro)/TLE, a global developmental corepressor, in these pathways. In particular, we discuss Gro/TLE's role at the junction between these two signal transduction cascades.
Keywords: EGFR, Groucho/TLE, Notch, signal transduction, transcriptional regulation
A handful of signalling pathways are repeatedly used to generate the innumerable varied cell types that comprise the bodies of all multicellular organisms. Mounting evidence suggests that the genesis of cellular complexity by so few pathways relies to a large extent on the crosstalk between them, that is, on the integration of signals transduced by these cascades, and on their combinatorial activities. For crosstalk to occur, whether with synergistic or antagonistic outcomes, signalling pathways must act on common cellular targets. In the nucleus, for example, diverse signalling pathways often impinge on a given promoter, which thus integrates the multiple cues required for stringent control of that specific gene. Recent findings from Drosophila now suggest that inputs from signalling pathways also converge on general transcriptional coregulators, in a way that can alter gene expression programmes involving large arrays of genes. Below, we focus on the antagonism between the Notch (N) and the epidermal growth factor receptor (EGFR) pathways, and on the role played by the universal transcriptional corepressor Groucho (Gro) as a focal point for cross-pathway regulation.
THE NOTCH PATHWAY
The highly conserved N signalling pathway participates in multiple developmental processes that entail cell fate determination in both invertebrates and higher organisms. This pathway is perhaps best known for its role in ‘lateral inhibition’, a biological setting in which the N cascade allows a signal emanating from one cell to alter the differentiation state of its initially equivalent neighbours. It thus acts to prevent groups of equipotent cells from all acquiring identical fates, thereby ensuring cellular diversity. In brief, the binding of one of two ligands, Serrate or Delta (Jagged1/2 and Delta1/3/4 in vertebrates, respectively), to the N receptor triggers a proteolytic cleavage of N, releasing its intracellular domain (NICD) and allowing it to shuttle into the nucleus. In the nuclear compartment, NICD associates with the DNA-binding transcription factor Suppressor of Hairless (Su(H); CBF1/RBP-Jk in vertebrates), and jointly they transduce the N signal by directly activating target gene expression (Figure 1A; Artavanis-Tsakonas et al, 1999; Lai, 2004). Prominent among the targets of the Su(H):NICD complex are the Enhancer of split (E(spl)) gene products, which are the ultimate executioners of the N signalling cascade and are therefore considered to be its major effectors. The E(spl) proteins (HES in vertebrates) are transcriptional repressors that have been best characterised in the context of neurogenesis as suppressors of proneural gene expression.
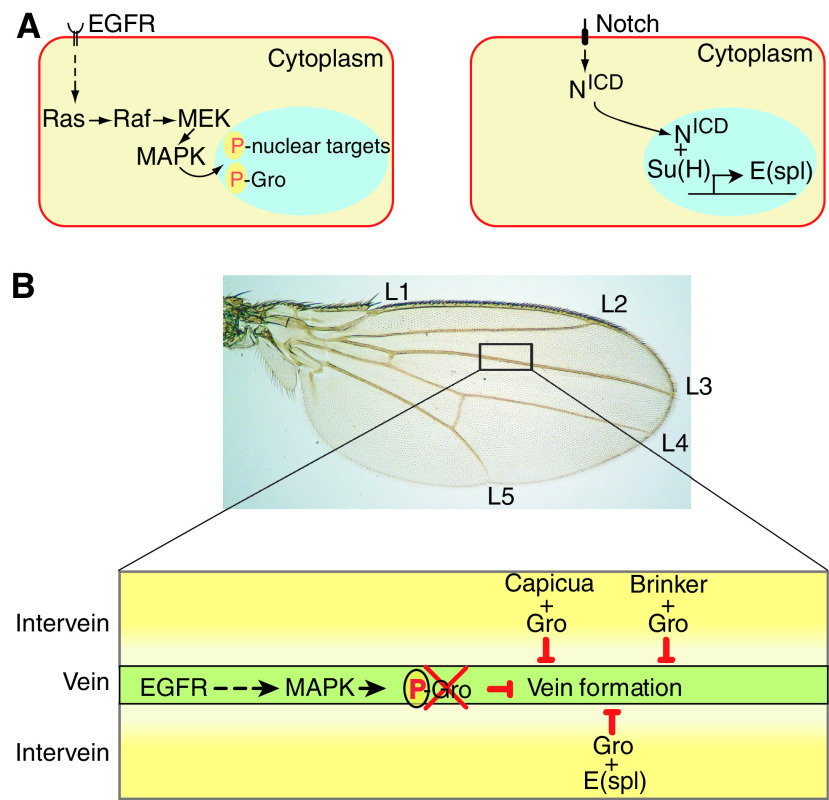
Figure 1.
EGFR signalling leads to the formation of wing veins by overriding Groucho-mediated repression. (A) Shown are abbreviated presentations of the EGFR and Notch signalling pathways. (B) A photograph of a Drosophila wing, with the veins numbered (L1–L5). Below, a schematic illustration of adjacent vein and intervein regions, depicting how MAPK phosphorylation of Gro may relieve transcriptional silencing by multiple antivein Gro-dependent repressors, promoting the formation of wing veins.
GROUCHO: A NUCLEAR EFFECTOR OF NOTCH AND OTHER SIGNALLING PATHWAYS
Importantly, the E(spl) proteins rely on the general corepressor Gro for silencing transcription of their targets, implicating Gro as a key effector of N signalling (e.g. Paroush et al, 1994; Giagtzoglou et al, 2003). Drosophila Gro is the founding member of a family of highly conserved metazoan corepressors that associate with a myriad of transcription factors, rendering them transcriptional repressors (Chen and Courey, 2000). Notably, the vertebrate homologues of Gro – the Transducin-Like Enhancer of split (TLE) and the Gro-related gene (Grg) proteins – similarly play transcriptional regulatory roles in diverse developmental stages of higher organisms, including in neurogenesis (Fisher and Caudy, 1998; Chen and Courey, 2000).
Interestingly, in the context of the N pathway Gro associates not only with the E(spl) repressors but also with Su(H) itself, allowing the latter to maintain N target genes in a silenced state in the absence of N signalling (Lai, 2004). For lack of space, in this minireview we only focus on Gro's role as a corepressor in conjunction with the E(spl) proteins, and on the developmental settings in which these transcriptional regulators partake.
Significantly, besides being components of the N pathway, the universal Gro/TLE corepressors have also been implicated as effectors of various other signalling cascades. For example, Gro mediates the repressor function of Brinker (Brk) and Tcf/Lef, the nuclear transducers of the Dpp/TGF-β and Wingless (Wg)/Wnt signalling pathways, respectively (Brantjes et al, 2001; Hasson et al, 2001; Zhang et al, 2001). Gro-dependent repressors also act downstream of receptor tyrosine kinase (RTK) pathways, for instance those mediated by the Torso receptor and by EGFR (Paroush et al, 1997; Price et al, 1997). Intriguingly, however, whereas Gro acts as a bona-fide component of the N, Wg/Wnt and Dpp/TGF-β pathways, as a coregulator that endows repressor potential on resident transcription factors affiliated to these cascades, it appears to function differently in the context of the EGFR and other RTK signalling pathways, at a novel level of regulation. Below, we assess evidence suggesting that, in effect, Gro-dependent repression is negated by EGFR signalling.
EGFR SIGNALLING DOWNREGULATES GROUCHO'S REPRESSOR ACTIVITY
The developmental roles played by the EGFR pathway and its involvement in intercellular communication and cell fate specification have been extensively reviewed elsewhere (Shilo, 2005). Briefly, activation of EGFR by its ligands leads to the relay of signals via the generic Ras/Raf/MEK/MAPK cascade, culminating in MAPK phosphorylation. Modified MAPK enters the nucleus and phosphorylates specific target transcription factors, thus linking signalling with gene expression regulation (Figure 1A).
It has lately emerged that Gro is a novel nuclear target for MAPK phosphorylation. Groucho contains two putative, evolutionarily conserved MAPK consensus sites, and is phosphorylated in cell culture and in vivo in response to EGFR signalling (Hasson et al, 2005). Importantly, the activation of EGFR, or of downstream components of the pathway such as Ras or MAPK, attenuates Gro-mediated repression in vivo. Conversely, when either EGFR or Ras are mutated, an opposite effect is observed, that is, Gro-mediated repression is strengthened (Hasson et al, 2005). Consistent with these results, repression is less potent by a derivative of Gro that mimics a constitutively pseudo-phosphorylated form (GroDD), in which the phospho-acceptor residues found in the two MAPK sites have been substituted for by negatively charged Aspartate (D) amino acids. Reciprocally, blocking phosphorylation of the MAPK sites by changing these residues to Alanine (A) (GroAA) leads to the overpotentiation of Gro's corepressor capacity (Hasson et al, 2005). Significantly, phosphorylation of Gro in response to MAPK activation hinders the E(spl) proteins – the nuclear effectors of the Notch pathway – from effectively silencing their target genes, as will be described below.
REGULATION OF GROUCHO-MEDIATED REPRESSION BY PHOSPHORYLATION
Phosphorylation of transcription factors stands out as an effective means by which developmental, mitogenic and metabolic cues alter gene expression in a rapid and reversible manner. Gro/TLE proteins contain putative phosphorylation sites for a number of kinases, and several studies have shown that Gro/TLE-mediated repression is affected by such modifications. Accordingly, Choi et al (2005) have recently reported that phosphorylation of Gro by DHIPK2 causes it to dissociate from one of its DNA-binding partners, bringing about derepression of target gene expression. In contrast, Stifani and co-workers have shown that casein kinase 2 phosphorylates Gro/TLE and enhances its repressor capacity, by augmenting its affinity to E(spl) proteins and to chromatin (Nuthall et al, 2002). Thus, differential and even opposing effects are brought about as a consequence of Gro/TLE phosphorylation by various kinases, presumably in response to different signals.
The findings assessed here suggest that Gro is also phosphorylated by MAPK, which attenuates its repressor capacity. How does this specific modification affect Gro activity? Theoretically, MAPK phosphorylation could have an impact on one, or even on several, of the many steps leading from the initial recruitment of Gro by DNA-binding partner proteins to, ultimately, transcriptional silencing. To date, little is known about the mechanism by which Gro/TLE proteins, once tethered to target promoters, block transcription of their targets. Clearly, homo-oligomerisation is a prerequisite for Gro/TLE repressor activity and, further, Gro/TLEs physically bind both histones and histone deacetylases (specifically, Rpd3/HDAC1) (e.g. Chen et al, 1999; Song et al, 2004). Collectively, these observations have led to the following model: Gro is initially tethered to specific gene regulatory regions via its associations with distinct DNA-bound repressors. Subsequently, additional Gro molecules are recruited by means of Gro:Gro and Gro:chromatin interactions. In this way, a complex formed by protein associations between a repressor and Gro may serve as a nucleation centre for regional repressive chromatin, that spreads some distance away from the initial repressor DNA-binding site, and likely accounts for Gro's long-range repression (Chen and Courey, 2000).
MAPK phosphorylation of Yan and Capicua (Cic), two Drosophila transcriptional repressors, leads to their nuclear export and degradation (Jimenez et al, 2000; Tootle et al, 2003); whether Gro is similarly affected is currently unknown. A future challenge would be to elucidate the precise molecular mechanism by which phosphorylation of Gro by MAPK downregulates its repressor potential, that is, does this modulation have an impact on Gro/TLE's homo-oligomerisation, subnuclear localisation, stability, or its interactions with other proteins such as DNA-bound repressors, histones or histone deacetylases?
ANTAGONISM BETWEEN THE EGFR AND NOTCH PATHWAYS: THE GROUCHO CONNECTION
The long-standing antagonistic relationship between the EGFR and Notch signalling pathways, and the opposing effects exerted by these signal transduction cascades, have been well documented in various developmental settings and organisms (Sundaram, 2005), yet the underlying molecular mechanism linking these pathways has remained poorly understood. As Gro/TLE is a well-established constituent of the N pathway, and given that in response to EGFR activation its repressor capacity is attenuated, it is feasible that this corepressor is at the heart of the crosstalk between these two pathways.
Two Drosophila developmental processes, in which the antagonism between the two cascades has previously been established and studied, are the prefiguring of the veins in the wing imaginal disc and of the mesothorax bristles in the adult fly. In these settings, the N pathway acts as an anti-vein and anti-bristle determinant and, in both cases, EGFR signalling functions to overcome the influence of the N pathway in a temporally and spatially restricted fashion, allowing veins and bristles to form (de Celis et al, 1997; Culi et al, 2001). Several lines of evidence now suggest that EGFR activity promotes vein and bristle formation at least in part by phosphorylating Gro and relieving E(spl)-mediated, Gro-dependent repression, and hence interfering with N signalling (Figure 1B). First, the misexpression of the pseudo-phosphorylated Gro-derivative (GroDD), the presumed end product of MAPK signalling vis-à-vis Gro, generates similar effects to those seen when the EGFR pathway is overactivated, that is, extra vein material and supernumerary bristles are formed (Hasson et al, 2005). Second, the overexpression of GroAA, the nonphosphorylatable derivative of Gro, renders the N pathway refractory to antagonistic EGFR activity, and the phenotypes caused by the misexpression of GroAA are comparable to those observed when the Notch pathway is ectopically activated, that is, loss of vein material and of bristles (de Celis et al, 1997; Culi et al, 2001; Hasson et al, 2005).
These results strongly suggest that some of the crosstalk between the N and EGFR pathways takes place at the level of the Gro/TLE corepressor. Crosstalk between these pathways could, of course, also occur at other levels. Significantly, MAPK regulation of Gro, which is at the junction between the two signalling cascades, provides an effective molecular mechanism that enables EGFR signalling to feed into the N cascade and to impinge on N transcriptional output.
ATTENUATION OF GROUCHO-MEDIATED REPRESSION BY EGFR SIGNALLING: POSSIBLE BIOLOGICAL IMPLICATIONS
The downregulation of Gro's repressor activity by EGFR signalling (and, likely, by other RTK pathways) has several possible biological implications. Below, we elaborate on three of these:
Coordinated derepression of a large array of genes
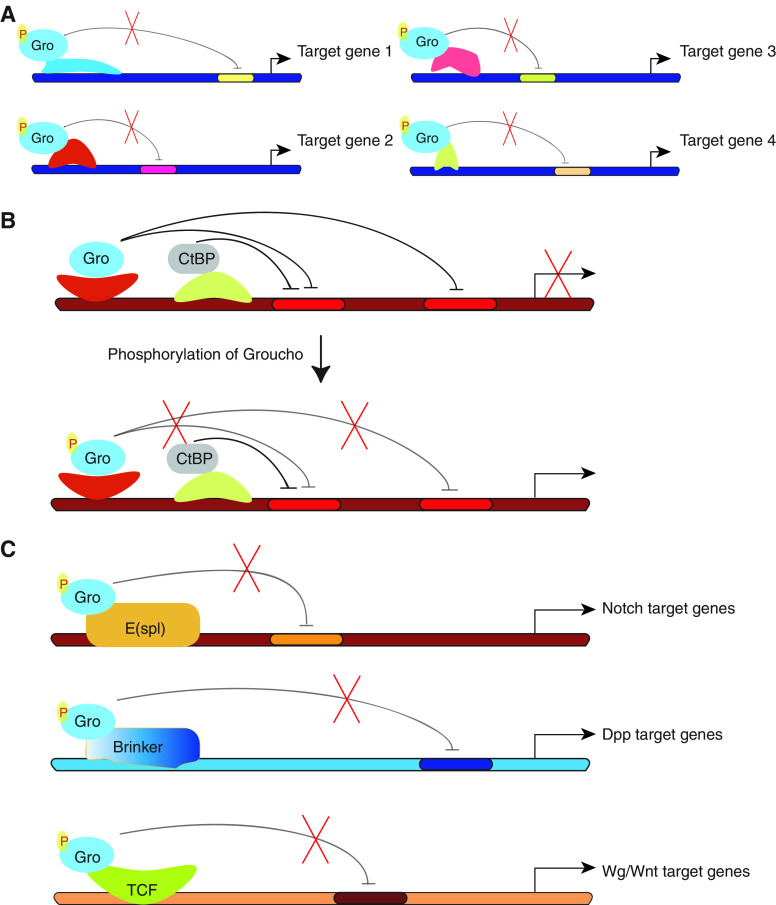
RTK signalling effectively alters cell fates, a phenomenon that entails eliciting extensive changes to gene expression programmes involving the transcription of numerous genes (e.g. Roberts et al, 2000), yet to date only a few transcription factors that are regulated by MAPK phosphorylation are known. How can such a dramatic change in gene expression profiles be achieved? Gro/TLE is a global corepressor, which acts together with manifold DNA-binding repressors, so it makes an ideal target for EGFR regulation. We propose that relief of Gro-dependent gene silencing, in response to RTK signals in distinct developmental settings, permits the coordinated derepression of a considerable number of genes giving rise to broad changes in gene expression profiles (Figure 2A).
Figure 2.
Attenuation of Groucho-mediated repression by RTK signalling: possible implications. (A) Coordinated derepression of a large array of genes. (B) Switching between modes of transcriptional regulation. (C) Groucho integrates RTK signaling with multiple pathways.
Wing vein patterning illustrates the above point. It turns out that besides the E(spl) proteins, there are at least two other Gro-dependent repressors that obstruct wing vein formation, namely Brk and Cic (Figure 1B; Roch et al, 2002; Cook et al, 2004). By targeting Gro, the coregulator that is shared between them, EGFR signalling can antagonise the entire group of repressors simultaneously, precluding them in a spatially and temporally regulated manner from exerting their repressor function over vein-making realisator genes. In this way, there is no need to independently regulate each and every anti-vein repressor individually.
Switching between modes of transcriptional regulation
The downregulation of Gro activity by RTK signalling may be a prerequisite for the transition from gene repression to activation. An increasing number of transcription factors appear to be bimodal in nature, in that on the one hand they act as Gro-dependent repressors, yet on the other hand they also activate gene expression in a context-dependent manner. Strikingly, the Gro-recruitment motifs in some of these transcription factors do not conform to any of the previously defined Gro-binding motifs or, in others, they bind Gro with a lower affinity. An elegant study of Lozenge, a factor belonging to this latter group, showed that when its weak Gro-recruitment motif (the tetrapeptide WRPY) is changed to a stronger Gro-binding motif (WRPW), this protein no longer alternates between its two modes of gene regulation; rather, it now behaves as a constitutive repressor (Canon and Banerjee, 2003). Conversely, and along this same line, we propose that in cells in which the available pool of ‘active’ Gro is lowered in response to RTK signalling, Lozenge and other bi-functional factors containing relatively weak Gro-binding domains will no longer repress, tilting the balance towards transcriptional activation.
RTK signalling could also be at the basis of the switch between long- and short-range repression. It appears that many repressors possess more than one repression domain. Brk and Su(H), for example, rely both on Gro as well as on a second corepressor called CtBP for turning off gene expression (Hasson et al, 2001). Gro and CtBP act qualitatively differently, in that Gro mediates repression over long distances (>1 Kb), whereas CtBP functions at short range (100–150 bp) (Figure 2B; Zhang and Levine, 1999). We propose that phosphorylation is a means of abating Gro-mediated repression, allowing repression that is CtBP-dependent (by Brk, Su(H) and/or other repressors) to dominate (Figure 2B). The necessity for such a mechanism is stressed by the fact that most promoters in multicellular organisms are compound, comprised of several stage- and tissue-specific enhancers that contain multiple binding sites for an assortment of transcriptional regulators. Gro-dependent repression should have a regional, dominant effect on adjacent enhancer elements, whereas CtBP acts locally. Thus, RTK signalling could be at the basis of the transition between these two modes of repression. In this scenario, MAPK phosphorylation will override Gro's long-range effect and, in this way, unmask CtBP's proximal repressor capability, allowing enhancer autonomy.
In summary, phosphorylation of Gro can provide a dynamic molecular switch that alternates between transcriptional repression and activation, or between different modes of repression.
Groucho integrates RTK signalling with multiple pathways
RTK pathways have frequently been shown to interact with other signal transduction pathways, consistent with extensive dialogues taking place between signalling cascades, yet the precise level(s) at which these pathways interconnect have remained largely elusive (Voas and Rebay, 2004). In a like manner to the case of the N and EGFR pathways, Gro/TLE could be a focal point between RTKs and other pathways. As mentioned above, Gro/TLE-dependent repressors function not only downstream of N, but also as effectors of the Dpp/TGF-β and Wg/Wnt pathways. The regulation of Gro by RTK signalling is expected to similarly affect the transcriptional output of these cascades (Figure 2C) (Hasson et al, 2005; our unpublished data).
Needless to say, crosstalk between pathways also takes place at other focal points. As mentioned above, target promoters frequently integrate distinct signals in the nucleus. A nice example for this is the expression of D-Pax2 in the Drosophila eye, that depends on the combined binding of effectors of both the N and EGFR pathways, together with tissue-specific transcription factors (Flores et al, 2000). Some of the intersections between signalling cascades might also occur via mutual cytoplasmic components. For instance, the Shaggy/GSK3 kinase is shared by both the Wg and the Hedgehog pathways (Jia et al, 2002).
CONCLUDING REMARKS
A general coregulator such as Gro/TLE makes a perfect integrator of inputs from RTK signal transduction cascades with multiple signalling networks. Remarkably, post-transcriptional phosphorylation also modulates the activity of several other global cofactors. For example, CtBP activity is modified by HIPK2 phosphorylation (Zhang et al, 2003), and HDACs such as Rpd3 have also been shown to be phosphorylated by MAPK in response to cellular stress (De Nadal et al, 2004). Thus, it is conceivable that, in addition to Gro, other widely used cofactors play a similar integrative role in cell signalling. It is also likely that similar interactions between RTKs and other pathways exist in vertebrates in general, and in neoplastic cells in particular, at the level of Gro/TLE and other general coregulators.
Acknowledgments
We thank our following colleagues for fruitful discussions and for valuable comments on the manuscript: Howard Cedar, Einat Cinnamon, Oded Meyuhas, Benny Shilo and Joel Yisraeli. We apologise to our many colleagues for the incomplete referencing due to rigid space constraints. Work in our laboratory is supported by Grants from the Israel Science Foundation, Israel Cancer Research Fund and the Król Charitable Foundation. PH is an EMBO Long-Term Postdoctoral Fellow and ZP a Braun Lecturer.
References
- Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776 [DOI] [PubMed] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H (2001) All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res 29: 1410–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canon J, Banerjee U (2003) In vivo analysis of a developmental circuit for direct transcriptional activation and repression in the same cell by a Runx protein. Genes Dev 17: 838–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Courey AJ (2000) Groucho/TLE family proteins and transcriptional repression. Gene 249: 1–16 [DOI] [PubMed] [Google Scholar]
- Chen G, Fernandez J, Mische S, Courey AJ (1999) A functional interaction between the histone deacetylase Rpd3 and the corepressor groucho in Drosophila development. Genes Dev 13: 2218–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CY, Kim YH, Kim YO, Park SJ, Kim EA, Riemenschneider W, Gajewski K, Schulz RA, Kim Y (2005) Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J Biol Chem 280: 21427–21436 [DOI] [PubMed] [Google Scholar]
- Cook O, Biehs B, Bier E (2004) brinker and optomotor-blind act coordinately to initiate development of the L5 wing vein primordium in Drosophila. Development 131: 2113–2124 [DOI] [PubMed] [Google Scholar]
- Culi J, Martin-Blanco E, Modolell J (2001) The EGF receptor and N signalling pathways act antagonistically in Drosophila mesothorax bristle patterning. Development 128: 299–308 [DOI] [PubMed] [Google Scholar]
- de Celis JF, Bray S, Garcia-Bellido A (1997) Notch signalling regulates veinlet expression and establishes boundaries between veins and interveins in the Drosophila wing. Development 124: 1919–1928 [DOI] [PubMed] [Google Scholar]
- De Nadal E, Zapater M, Alepuz PM, Sumoy L, Mas G, Posas F (2004) The MAPK Hog1 recruits Rpd3 histone deacetylase to activate osmoresponsive genes. Nature 427: 370–374 [DOI] [PubMed] [Google Scholar]
- Fisher AL, Caudy M (1998) Groucho proteins: transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates and invertebrates. Genes Dev 12: 1931–1940 [DOI] [PubMed] [Google Scholar]
- Flores GV, Duan H, Yan H, Nagaraj R, Fu W, Zou Y, Noll M, Banerjee U (2000) Combinatorial signaling in the specification of unique cell fates. Cell 103: 75–85 [DOI] [PubMed] [Google Scholar]
- Giagtzoglou N, Alifragis P, Koumbanakis KA, Delidakis C (2003) Two modes of recruitment of E(spl) repressors onto target genes. Development 130: 259–270 [DOI] [PubMed] [Google Scholar]
- Hasson P, Egoz N, Winkler C, Volohonsky G, Jia S, Dinur T, Volk T, Courey AJ, Paroush Z (2005) EGFR signaling attenuates Groucho-dependent repression to antagonize Notch transcriptional output. Nat Genet 37: 101–105 [DOI] [PubMed] [Google Scholar]
- Hasson P, Muller B, Basler K, Paroush Z (2001) Brinker requires two corepressors for maximal and versatile repression in Dpp signalling. Embo J 20: 5725–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Amanai K, Wang G, Tang J, Wang B, Jiang J (2002) Shaggy/GSK3 antagonizes Hedgehog signalling by regulating Cubitus interruptus. Nature 416: 548–552 [DOI] [PubMed] [Google Scholar]
- Jimenez G, Guichet A, Ephrussi A, Casanova J (2000) Relief of gene repression by torso RTK signaling: role of capicua in Drosophila terminal and dorsoventral patterning. Genes Dev 14: 224–231 [PMC free article] [PubMed] [Google Scholar]
- Lai EC (2004) Notch signaling: control of cell communication and cell fate. Development 131: 965–973 [DOI] [PubMed] [Google Scholar]
- Nuthall HN, Husain J, McLarren KW, Stifani S (2002) Role for Hes1-induced phosphorylation in Groucho-mediated transcriptional repression. Mol Cell Biol 22: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroush Z, Finley Jr RL, Kidd T, Wainwright SM, Ingham PW, Brent R, Ish-Horowicz D (1994) Groucho is required for Drosophila neurogenesis, segmentation, and sex determination and interacts directly with hairy-related bHLH proteins. Cell 79: 805–815 [DOI] [PubMed] [Google Scholar]
- Paroush Z, Wainwright SM, Ish-Horowicz D (1997) Torso signalling regulates terminal patterning in Drosophila by antagonising Groucho-mediated repression. Development 124: 3827–3834 [DOI] [PubMed] [Google Scholar]
- Price JV, Savenye ED, Lum D, Breitkreutz A (1997) Dominant enhancers of Egfr in Drosophila melanogaster: genetic links between the Notch and Egfr signaling pathways. Genetics 147: 1139–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Nelson B, Marton MJ, Stoughton R, Meyer MR, Bennett HA, He YD, Dai H, Walker WL, Hughes TR, Tyers M, Boone C, Friend SH (2000) Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science 287: 873–880 [DOI] [PubMed] [Google Scholar]
- Roch F, Jimenez G, Casanova J (2002) EGFR signalling inhibits Capicua-dependent repression during specification of Drosophila wing veins. Development 129: 993–1002 [DOI] [PubMed] [Google Scholar]
- Shilo BZ (2005) Regulating the dynamics of EGF receptor signaling in space and time. Development 132: 4017–4027 [DOI] [PubMed] [Google Scholar]
- Song H, Hasson P, Paroush Z, Courey AJ (2004) Groucho oligomerization is required for repression in vivo. Mol Cell Biol 24: 4341–4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram MV (2005) The love-hate relationship between Ras and Notch. Genes Dev 19: 1825–1839 [DOI] [PubMed] [Google Scholar]
- Tootle TL, Lee PS, Rebay I (2003) CRM1-mediated nuclear export and regulated activity of the receptor tyrosine kinase antagonist YAN require specific interactions with MAE. Development 130: 845–857 [DOI] [PubMed] [Google Scholar]
- Voas MG, Rebay I (2004) Signal integration during development: insights from the Drosophila eye. Dev Dyn 229: 162–175 [DOI] [PubMed] [Google Scholar]
- Zhang H, Levine M (1999) Groucho and dCtBP mediate separate pathways of transcriptional repression in the Drosophila embryo. Proc Natl Acad Sci USA 96: 535–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Levine M, Ashe HL (2001) Brinker is a sequence-specific transcriptional repressor in the Drosophila embryo. Genes Dev 15: 261–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yoshimatsu Y, Hildebrand J, Frisch SM, Goodman RH (2003) Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell 115: 177–186 [DOI] [PubMed] [Google Scholar]