Abstract
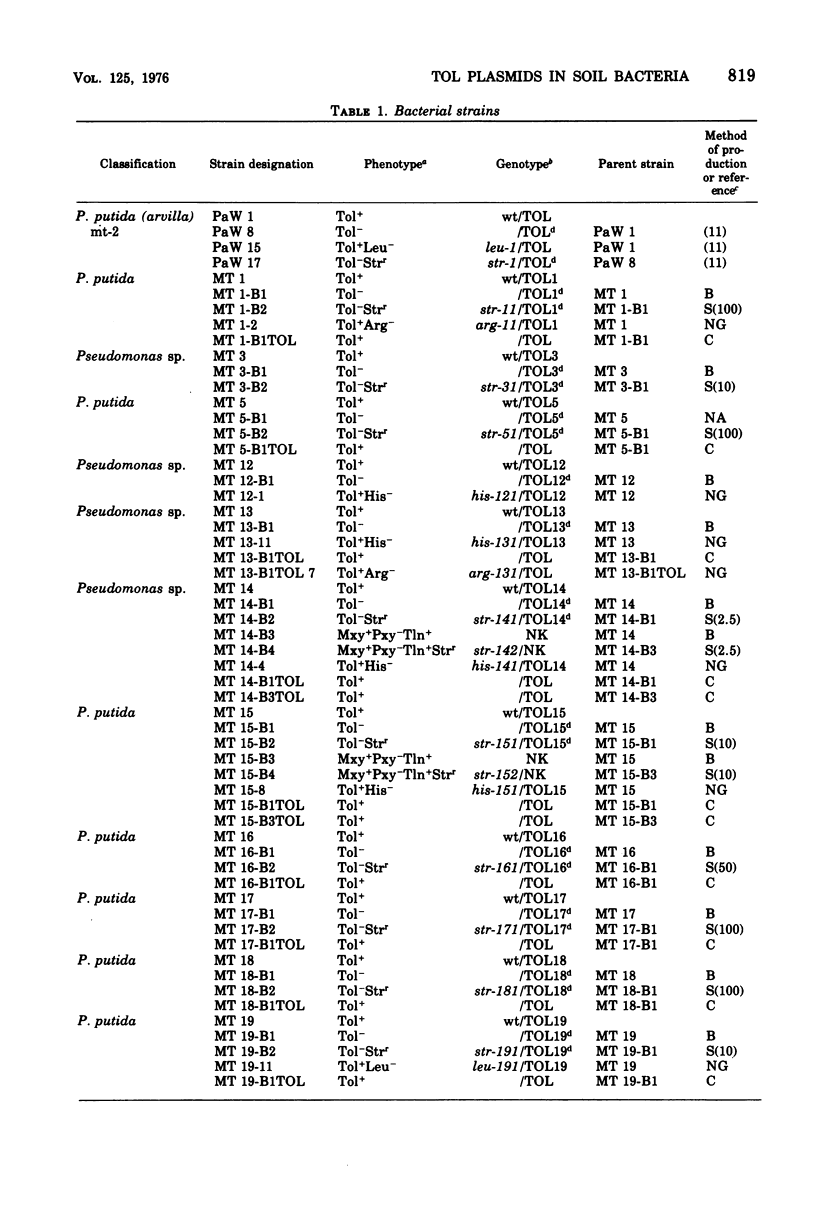
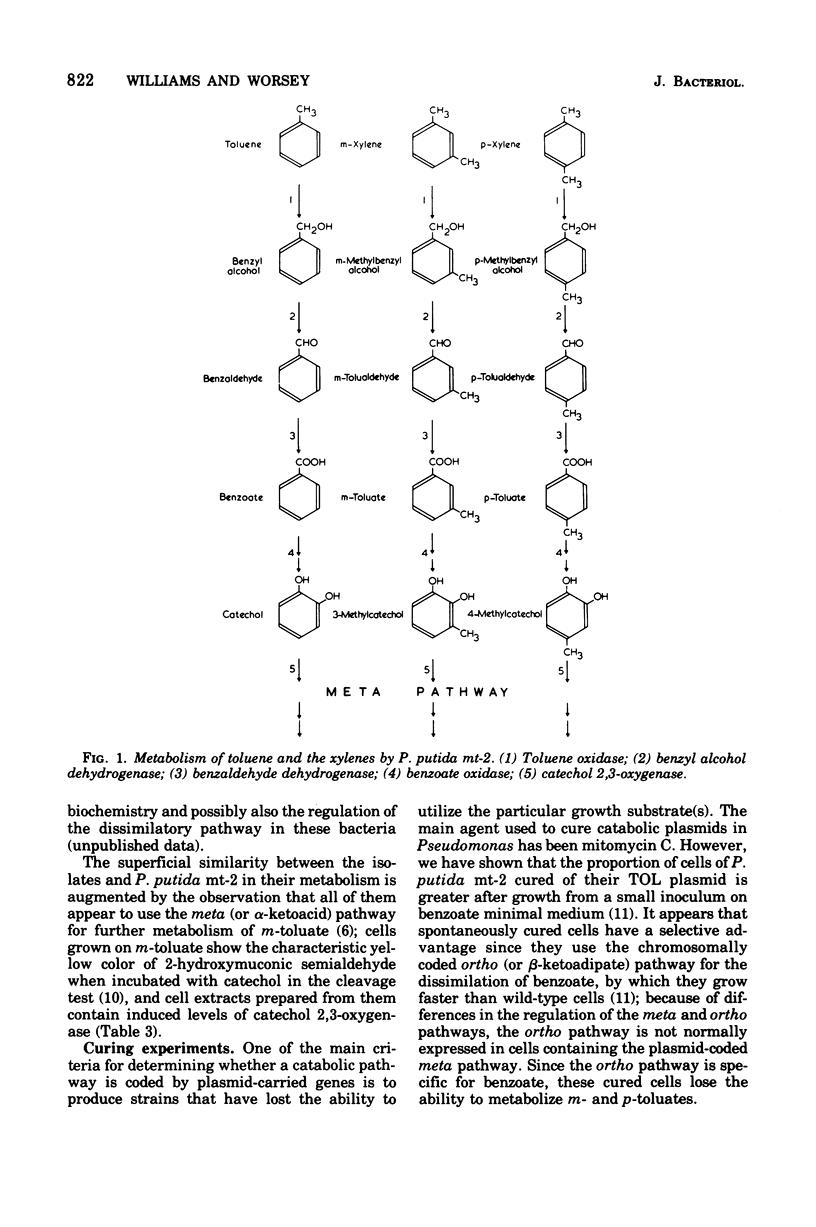
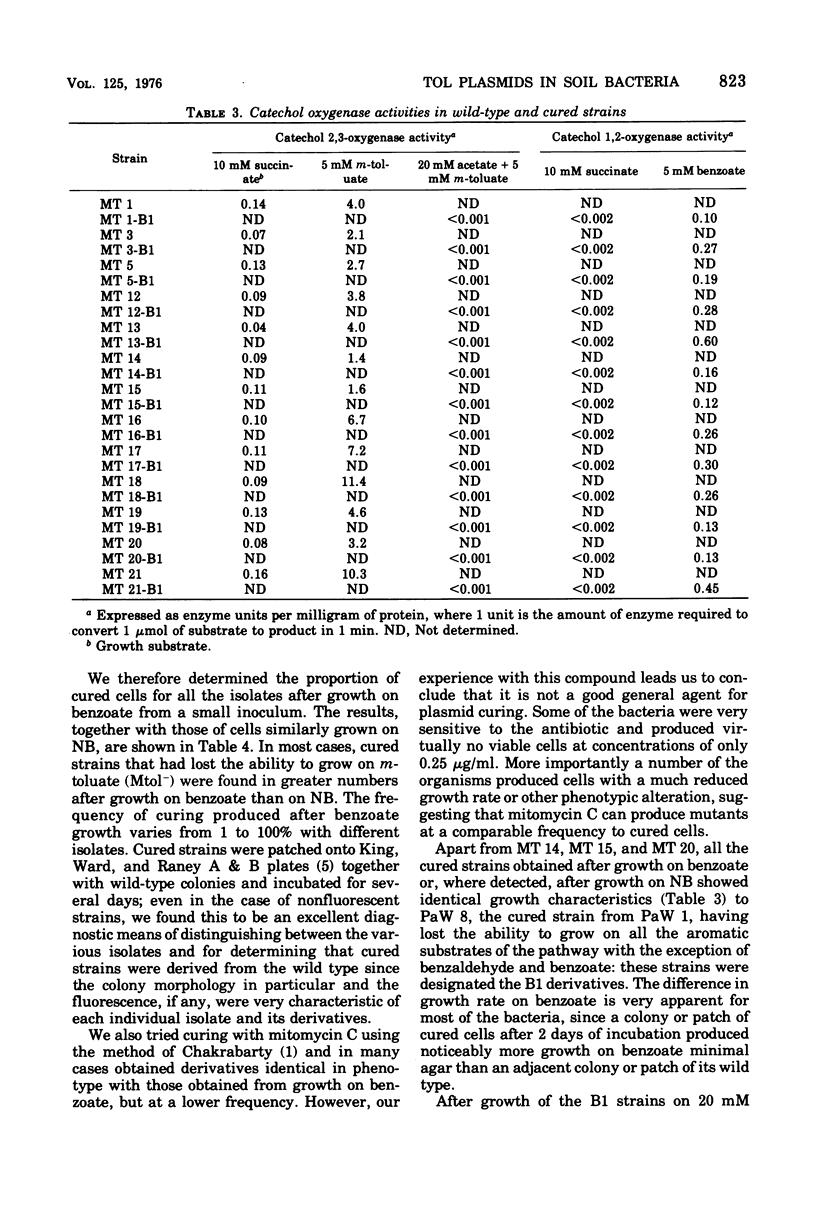
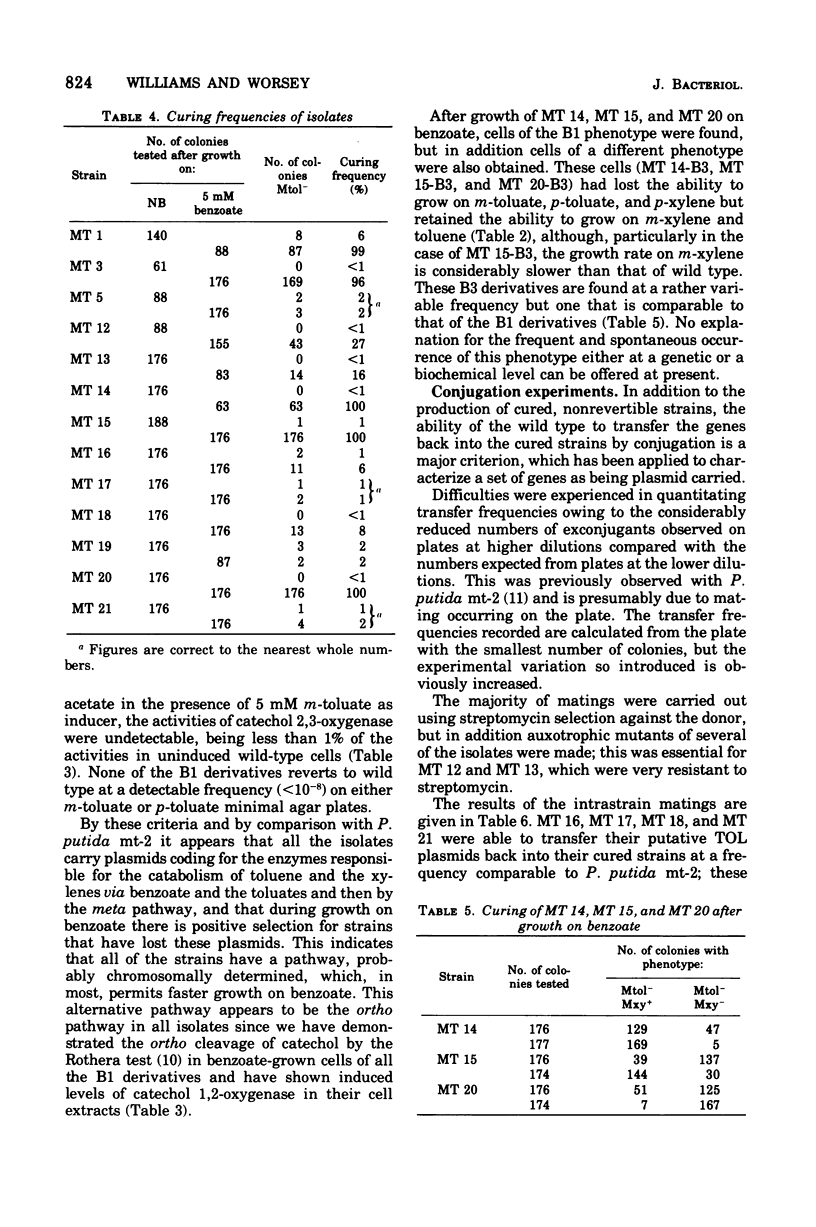
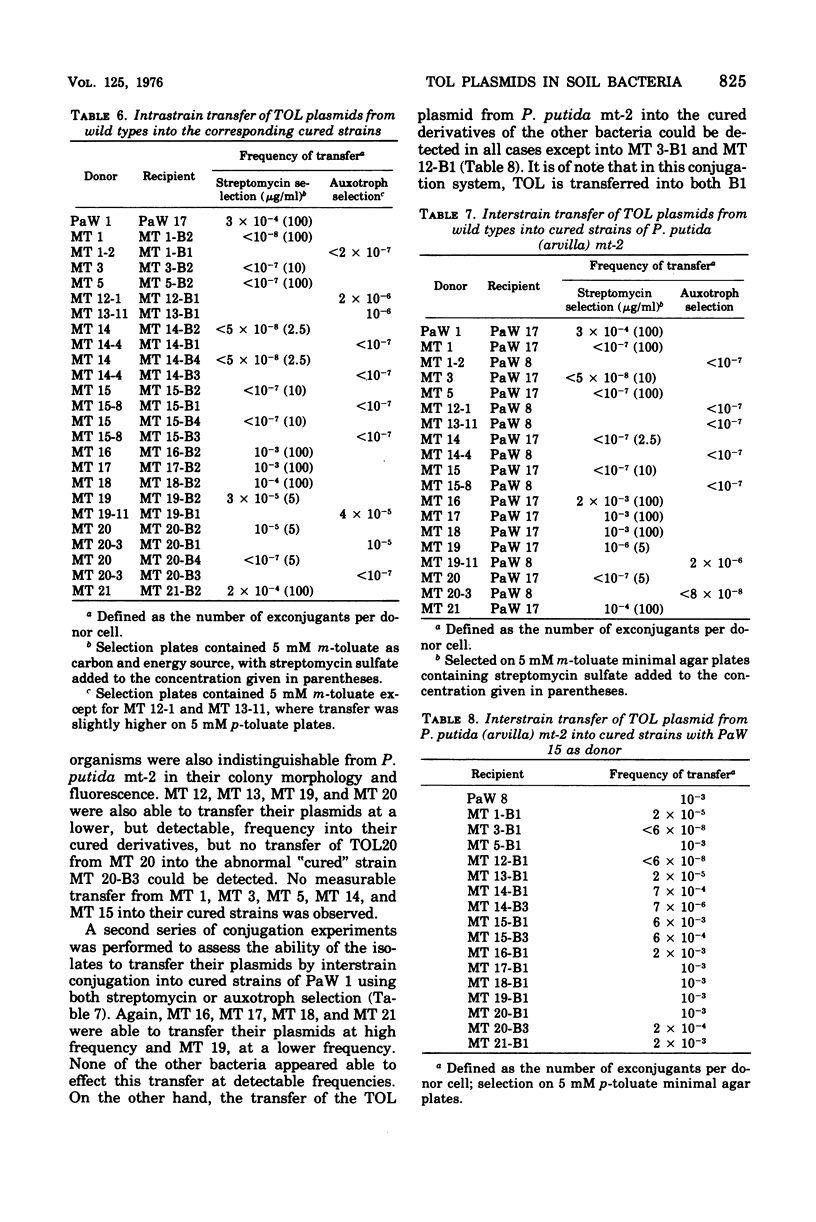
Thirteen bacteria have been isolated from nine different soil samples by selective enrichment culture on m-toluate (m-methylbenzoate) minimal medium. Eight of these were classified as Pseudomonas putida, one as a fluorescent Pseudomonas sp., and four as nonfluorescent Pseudomonas sp. All 13 strains appeared to carry TOL plasmids superficially similar to that previously described in P. putida mt-2 in that: (i) all the wild-type strains could utilize toluene, m-xylene, and p-xylene as sole carbon and energy sources, (ii) these growth substrates were metabolized through the corresponding alcohols and aldehydes to benzoate, m-toluate, and p-toluate, respectively, and thence by the divergent meta (or alpha-ketoacid) pathway, and (iii) the isolates could simultaneously and spontaneously lose their ability to utilize the hydrocarbons, alcohols, aldehydes, and acids, particularly during growth on benzoate, giving rise to cured strains which could grow only on benzaldehyde and benzoate of the aromatic substrates by the alternative ortho (or beta-ketoadipate) pathway. Eight of the isolates were able to transfer their TOL plasmids into their own cured strains, but only five were able to transfer them in interstrain conjugation into the cured strains, but only five were able to transfer them in interstrain conjugation into the cured derivative of P. putida mt-2. However, P. putida mt-2 was able to transfer its TOL plasmid into 11 of the cured isolates, and eight of these were able to retransmit this foreign plasmid in intrastrain conjugation with their own cured derivatives. Three of the isolates, MT 14, MT 15, and MT 20, differed significantly from the others in that the wild-type strains dissimilated the p-methyl-substituted substrates poorly, and also, during growth on benzoate, in addition to the cured derivatives, they gave rise to derivatives with a phenotype intermediate between the cured and wild-type strains, the biochemical and genetic nature of which has not been elucidated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic basis of the biodegradation of salicylate in Pseudomonas. J Bacteriol. 1972 Nov;112(2):815–823. doi: 10.1128/jb.112.2.815-823.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M. Genetic fusion of incompatible plasmids in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1641–1644. doi: 10.1073/pnas.70.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Murray K., Duggleby C. J., Sala-Trepat J. M., Williams P. A. The metabolism of benzoate and methylbenzoates via the meta-cleavage pathway by Pseudomonas arvilla mt-2. Eur J Biochem. 1972 Jul 24;28(3):301–310. doi: 10.1111/j.1432-1033.1972.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Rheinwald J. G., Chakrabarty A. M., Gunsalus I. C. A transmissible plasmid controlling camphor oxidation in Pseudomonas putida. Proc Natl Acad Sci U S A. 1973 Mar;70(3):885–889. doi: 10.1073/pnas.70.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. L., Dunn N. W. Transmissible plasmid coding for the degradation of benzoate and m-toluate in Pseudomonas arvilla mt-2. Genet Res. 1974 Apr;23(2):227–232. doi: 10.1017/s0016672300014853. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


