Abstract
The human transcription factor B-TFIID is comprised of TATA-binding protein (TBP) in complex with one TBP-associated factor (TAF) of 170 kDa. We report the isolation of the cDNA for TAFII170. By cofractionation and coprecipitation experiments, we show that the protein encoded by the cDNA encodes the TAF subunit of B-TFIID. Recombinant TAFII170 has (d)ATPase activity. Inspection of its primary structure reveals a striking homology with genes of other organisms, yeast MOT1, and Drosophila moira, which belongs to the Trithorax group. Both homologs were isolated in genetic screens as global regulators of pol II transcription. This supports our classification of B-TFIID as a pol II transcription factor and suggests that specific TBP–TAF complexes perform distinct functions during development.
Keywords: RNA polymerase II, dATPase activity, Trithorax
RNA synthesis in the eukaryotic nucleus is catalyzed by three distinct RNA polymerases (pol I, II, and III) and requires in each case auxiliary initiation factors. The TATA-binding protein (TBP) was first identified as the DNA-binding subunit of transcription factor (TF) IID. Subsequently, TBP was found to perform essential roles in the pol I and III systems. The combination with other proteins (TBP-associated factors, TAFs) dictates the polymerase specificity of the TBP complex (for review see ref. 1). The TFIID complex harbors more than eight TAFs ranging in size between 18 and 250 kDa, resulting in a complex of >700 kDa. In in vitro assays measuring TFIID activity, it was found that TAFs play an essential role in transcription activation (for review see ref. 2). Coactivator function of TAFs is underscored by observations that individual TAFs bind different sets of activation domains and that this correlates with activation domain-specific stimulation of transcription (3, 4). In addition, specific TBP–TAF subassemblies can discriminate between different core promoters (5). The exact stoichiometry of TAFs in the TFIID complex has not been established. Certain TAFs appear to be less abundant than others, and this may relate to partial dissociation of TFIID during isolation or to the existence of different TFIID complexes in the cell (6). The latter may be explained by a model that TAFs are assembled after TBP association with the promoter (1). In this respect, it is important to note that TBP does not exist as a monomer in mammalian cell extracts (7).
A comparison of TAF genes cloned from human, Drosophila, and yeast revealed a high degree of structural conservation (for review see ref. 2). Genetic analyses in yeast indicated that, as expected, genes encoding TAFs are essential for viability. However, additional experiments showed that transcription activation can occur in TAF-depleted yeast cells (8, 9). In addition, analysis of pol II holoenzyme-directed transcription indicated that in vitro activation can occur in the absence of TAFs (10). Together, these reports indicate that TAFs are not generally required for transcription activation in yeast.
We have found that, in mammalian cell extracts, the majority of TBP is present in the pol II initiation factor B-TFIID. This factor is comprised of TBP and a protein of 170 kDa, TAFII170 (11), and can support basal transcription in transcription assays measuring TFIID activity. Of interest, transcription with B-TFIID does not respond to the activators tested (7). This indicates that the B-TFIID complex is unable to establish activator contacts, which are involved in the coactivator function of TAF components of TFIID. B-TFIID binds with less stability to the TATA box, and this property may relate to its inability to commit a template for transcription, which is in contrast to TBP or TFIID (7). In addition, B-TFIID has a potent (d)ATPase activity (11). Pugh and coworkers (12) reported that a TAF of 172 kDa is part of the pol III factor TFIIIB and proposed that it is equivalent to the TAFII170 component of B-TFIID. However, other groups did not observe a TAF of 170 kDa in TFIIIB (13, 14). In addition, analysis of B-TFIID indicated that it does not function in pol III transcription assays (15). Nevertheless, it remains possible that TAFII170 is present in both B-TFIID and TFIIIB factors.
In this study, we report the cloning of the TAFII170 cDNA and its copurification with B-TFIID. In addition, we show that recombinant TAFII170 protein associates with recombinant TBP and has considerable (d)ATPase activity. The primary structure of human TAFII170 identifies two global regulators of pol II transcription as homologs in other organisms, which supports the role of B-TFIID as a pol II factor.
MATERIALS AND METHODS
Cloning of TAFII170 cDNA.
The EST clone encoded amino acids 1616–1699 of TAFII170 and was used to screen a human B cell cDNA library following standard procedures (16). Positive clones were sequenced on both strands using the T7 sequencing kit (Pharmacia). The two longest clones (N5D and 3N10) contained a nonspliced intron. This was corrected by reverse transcriptase-PCR using HeLa cell mRNA. The PCR fragment was rebuilt into the 3N10 clone after sequence verification. This cDNA lacks the first 16 codons. A mouse fibroblast cDNA library (#1023, CLONTECH) was screened with a 360-bp fragment encoding amino acids 17–153. This resulted in one positive clone, M5A, which was sequenced and used to isolate a human genomic fragment carrying the ATG and the missing codons. The genomic and cDNA sequences of human TAFII170 were combined, and the DNA sequence of the human TAFII170 coding and untranslated regions has been deposited in the GenBank database under accession number AJ001017.
Purification of B-TFIID and Recombinant TAFII170.
Protein fractions containing B-TFIID [B] or TFIID [D] were obtained by phosphocellulose chromatography of HeLa whole cell extract as described (7). B-TFIID was purified by phosphocellulose, Q-Sepharose, and MonoS chromatographic steps essentially as described (11). Subsequently, B-TFIID fractions were adjusted to buffer H [10% (vol/vol) glycerol/50 mM KCl/50 μM CaCl2/0.5 mM phenylmethylsulfonyl fluoride/0.5 mM DTT/0.2 mM sodium bisulfite/1 μg/ml aprotinin/1 μg/ml leupeptin] plus 10 mM potassium phosphate (pH 7.0) and applied to a 5-ml hydroxylapatite column (Bio-Rad). The column was developed with a linear gradient from 10 to 250 mM potassium phosphate (pH 7.0). B-TFIID-containing fractions (140–200 mM potassium phosphate) were dialyzed to buffer A [20 mM Hepes⋅KOH, pH 7.9/20% (vol/vol) glycerol/1 mM EDTA/DTT/protease inhibitors as above] plus 0.1 M KCl and applied to a 1-ml heparin HiTrap column (Pharmacia). After washing with buffer A plus 0.2 M KCl, B-TFIID was step-eluted with buffer A plus 0.45 M KCl. This B-TFIID fraction was analyzed by gel filtration as described (7).
Recombinant TAFII170 vaccinia viruses were generated as described earlier (17) by homologous recombination with pTAF–gptATA carrying the TAFII170 ORF, in which amino acids 1–16 were replaced by MGSSHHHHHHSSGLVPRGSHMAMGYPYDVPDYARSNS, which contains an 6x His tag to facilitate purification. For purification of recombinant TAFII170, Hela S3 cells (2.5·108) were infected as described (17) with TAFII170 virus at a multiplicity of infection of 3 plaque forming units per cell. After a 20-h infection at 37°C, cells were harvested. A whole cell extract was prepared and adjusted to buffer T [20 mM Tris⋅HCl, pH 7.9/20% (vol/vol) glycerol/0.5 mM phenylmethylsulfonyl fluoride/0.2 mM sodium bisulfite] plus 50 mM KCl/1 mM EDTA/1 mM DTT. The extract was applied to a 65-ml Q-Sepharose FF column, and, after washing with buffer T plus 0.15 M KCl/0.1 mM EDTA/1 mM β-mercaptoethanol (β-ME), TAFII170 protein was eluted with buffer T plus 0.45 M KCl/0.1 mM EDTA/1 mM β-ME. Imidazole was added to 1 mM, and this fraction was applied to a 2.5-ml NiNTA agarose column (Qiagen, Chatsworth, CA). The column was washed with buffer T plus 20 mM imidazole/50 mM KCl/0.1 mM EDTA/1 mM β-ME, and TAFII170 protein was eluted with buffer T plus 0.25 M imidazole/50 mM KCl/0.1 mM EDTA/1 mM β-ME. TAFII170 fractions were adjusted to the conductivity of buffer A plus 0.1 M KCl and applied to a 1-ml heparin HiTrap column. The column was developed by a linear gradient of 0.1–0.6 M KCl in buffer A. Recombinant TAFII170 elutes at 0.28 M KCl. TAFII170-containing fractions were identified by immunoblotting.
Immunological Analyses.
Polyclonal antisera were raised against an N-terminal region (17–154) or against a C-terminal region (1616–1699) of human TAFII170 as described (7). mAb 20C7 and 22D1 are directed against the N-terminal half of human TBP and were isolated according to standard procedures (18). For coimmunoprecipitation, protein fractions were incubated in ELB buffer [50 mM Hepes⋅KOH, pH 7.9/0.3 M KCl/5 mM MgCl2/0.5 mM EDTA/0.1% Nonidet P-40/1 mM β-ME and protease inhibitors as above] with antibody-coated protein A–agarose beads for 3 h at 4°C. The beads were washed, and immune complexes were analyzed on immunoblots, which were prepared and developed as described (18).
Coinfection by Recombinant TBP and TAFII170 Vaccinia Viruses.
For the coimmunoprecipitation experiment, A14 mouse fibroblast cells were infected by recombinant TAFII170 and/or recombinant TBP viruses at a multiplicity of infection of 3 (TAF) or 1 (TBP) plaque forming units per cell. After 20 h of infection at 37°C, cells were washed twice with PBS and lysed by incubation in ELB buffer for 30 min at 0°C. After removal of cell debris by centrifugation, lysates were used directly.
For the NiNTA–agarose affinity step, HeLa-TK− cells were infected with virus(es), and lysates were prepared as above in ELB buffer plus 1 mM imidazole and without EDTA. Lysates were applied to 1-ml NiNTA–agarose columns. After washing with ELB buffer plus 20 mM imidazole and without EDTA, bound proteins were eluted by increasing the imidazole concentration to 0.25 M in ELB buffer.
ATPase Assay.
ATPase activity was measured in reactions of 10 μl, which contained 40 mM Hepes⋅KOH, pH 8.05/2–4% (vol/vol) glycerol/25–35 mM KCl/0.2 mM EDTA/5 mM MgCl2/2 mM DTT/0.5 mM phenylmethylsulfonyl fluoride/0.2 mg/ml BSA and were started by the addition of 100 μM [α-32P] ATP. After a 2-h incubation at 30°C, part of the reaction was analyzed by thin layer chromatography on polyethyleneimine plates using 0.5 M LiCl/1 M HCOOH as eluens. Identity of the products was verified by UV shadowing of cochromatographed ATP and ADP.
RESULTS
Generation and Analysis of TAFII170 Antibodies.
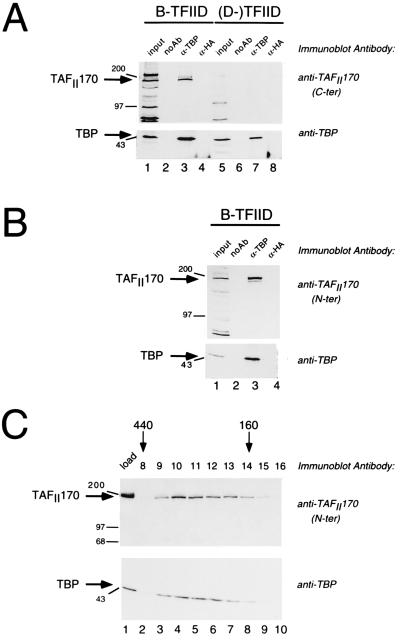
Microsequencing of TAFII170 protein is hampered by its low abundance in mammalian extracts (11). To circumvent this, we speculated that TAFII170 belongs to the SNF2 superfamily (19). This was based on its size and its apparent (d)ATPase activity (11). In addition, a member of this group, yeast Mot1p, was identified as a TAF by Weil and coworkers (20). Antisera against human members of the SNF2 family (ERCC-6, Etl-1, and an EST with homology to Mot1p) were obtained and tested for recognition of TAFII170 in immunoblot experiments with B-TFIID fractions. One of these sera recognized a protein band with the mobility of TAFII170. This serum was raised against an human EST clone with homology to a C-terminal region of yeast Mot1p (1612–1691). If the protein recognized by this antiserum is indeed TAFII170, it should be immunoprecipitated from B-TFIID fractions by antibodies directed to TBP. This was investigated using [B] or [D] fractions from the phosphocellulose chromatographic step, which separates B-TFIID (in [B]) from traditional (D-)TFIID (in [D]) (7). Precipitated proteins were analyzed for TBP and TAFII170 content by immunoblot analysis. Fig. 1A shows that the protein recognized by the antiserum is very efficiently precipitated by TBP antibodies but not by control antibodies (lanes 3 and 4). As expected, no protein bands are recognized in the anti-TBP precipitate of the (D-)TFIID fraction (Fig. 1A, lane 7). This indicates that the EST clone used to generate the antibodies is part of the human cDNA for the TAFII170 protein and prompted us to isolate the remainder of the cDNA (see below).
Figure 1.
TAFII170 is recognized by antisera raised against N- or C-terminal regions of the protein encoded by the isolated cDNA. (A) Immunoprecipitations using isolated B-TFIID or D-TFIID fractions were performed without antibody (lanes 2 and 6), with TBP mAb 22D1 (lanes 3 and 7), or with 12CA5 antibody (lanes 4 and 8) attached to protein A–agarose beads. Lanes 1 and 5 contain 50% of the input [B] or [D] fraction. Immune complexes were analyzed on immunoblots with polyclonal antibodies raised against the C terminus of TAFII170 (Upper) and, subsequently, with the TBP mAb 20C7 (Lower). The arrows indicate the positions of TAFII170 and TBP. Positions of the markers (in kilodaltons) are indicated to the left. (B) The N-terminal antibodies also recognize TAFII170 in B-TFIID. [B] fraction was subjected to immunoprecipitation as in A. Lane 1 contains 30% of the input [B] fraction. No antibodies (lane 2), 22D1 (lane 3) or 12CA5 antibodies (lane 4) were coupled to protein A–agarose before incubation with [B]. Immunoblots were analyzed as in A. (C). TAFII170 protein coelutes with TBP on a gel filtration column. Four microliters of the load (lane 1) and 80 μl of fractions 8–16 (lanes 2–10) were analyzed on immunoblots with TAFII170 (Upper) and TBP antibodies (Lower) as indicated to the right. The arrows above the blot denote fractionation of standard proteins by their molecular mass.
The sequence of the EST clone is in a conserved region of the SNF2 superfamily. This raised the possibility that the antiserum was directed against another SNF2 family member but that it cross-reacted with TAFII170. Therefore, we generated antibodies against the most N-terminal region of one of the obtained cDNA clones. This region is not conserved in the SNF2 superfamily. The obtained antibodies were analyzed in a TBP coprecipitation experiment. This analysis showed that the protein recognized by the N-terminal antibodies is indeed immunoprecipitated efficiently by TBP antibodies from the B-TFIID fraction (Fig. 1B, lane 3). In a similar analysis with (D-)TFIID, this antiserum did not recognize coprecipitating proteins (data not shown).
To elaborate these findings, we analyzed fractions from the different steps of a B-TFIID purification with TAFII170 and TBP antibodies. A strict coelution of TBP and TAFII170 was observed (data not shown). B-TFIID peak fractions from the fifth chromatographic step were subjected to gel filtration analysis. As expected, immunoblotting analysis showed that TAFII170 and TBP coelute from this column (Fig. 1C). Both proteins peak in fractions 10 to 12, which correspond to a molecular size of 300 kDa. This is very similar to the earlier analysis of B-TFIID (7) and illustrates the stability of the TBP–TAFII170 interaction. Taken together, the immunoblotting experiments of Fig. 1 show that cDNA sequences used to generate the antibodies encode the human TAFII170 component of B-TFIID.
Analysis of the cDNA for Human TAFII170.
We obtained the complete ORF of human TAFII170, which encodes a 1849-amino acid protein with a calculated molecular mass of 206,771 Da. Whereas previous analysis indicated that the TAF component of B-TFIID has a relative mobility of 170 kDa (hence its name), TAFII170 migrated closer to its calculated molecular weight in our current gel system. To avoid confusion, we will continue to refer to the TAF subunit of human B-TFIID as TAFII170. As expected, the TAFII170 sequence harbors the seven motifs characteristic for proteins containing ATPase activity (Fig. 2). A database search with the TAFII170 ATPase region (amino acids 1263–1779) revealed 62% identity with yeast Mot1p (21) and 69% identity with Drosophila 89B helicase (22). In addition, this region is very similar to the ATPase regions of, for example, human ERCC-6, murine Etl-1, Drosophila brahma, and yeast Snf2p/Swi2p (37, 34, 35, and 33% identities, respectively).
Figure 2.
Amino acid sequence of TAFII170 and its alignment with Mot1p and 89B helicase sequences. Alignment was performed using the clustal w algorithm (25). Only the N-terminal 205 amino acids of mouse TAFII170 have been determined, and this part is included in the comparison. The sequence for Drosophila 89B helicase is derived from a partial cDNA clone, lacking the N-terminal half of the ORF. Immunoblotting experiments indicate that the full length 89B helicase protein has a molecular mass 210 kDa (22), which is similar to that of TAFII170. The boxed regions mark ATPase motifs and are indicated by roman numerals. Putative nuclear localization signals are indicated by bars. The asterisk denotes the mot1–24 mutation R1507K (30).
The homology among TAFII170, Mot1p, and 89B helicase extends beyond the ATPase region (Fig. 2). The first 1262 amino acids of TAFII170 are 26% identical to the corresponding region of Mot1p and 33% identical to amino acids 1–343 of 89B helicase [the N-terminal half of 89B helicase has not been cloned yet (22)]. Comparison of this TAFII170 region with other SNF2 family members reveals no significant homology. In conclusion, this analysis shows that TAFII170, Mot1p, and 89B helicase are encoded by homologous genes in different organisms. The Drosophila FlyBase web site (http://morgan.harvard.edu/htbin-post/gene.script?FBgn0002783) shows that the 89B helicase is encoded by the Trithorax gene moira (23).
Association of Recombinant TAFII170 with Recombinant TBP.
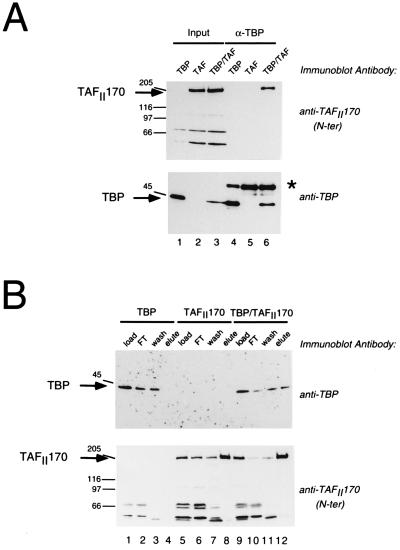
To investigate whether the recombinant TAFII170 can associate with TBP, we used the recombinant vaccinia virus system. The N terminus of TAFII170 was modified by addition of a His-tag and recombinant TAFII170 virus was generated. Mouse fibroblast cells were infected by this virus in combination with a TBP-expressing virus (kind gift of H. Stunnenberg). Extracts were prepared by mild lysis of the cells and subjected to immunoprecipitation with TBP antibodies. The results show that the TAFII170 protein is present in the immunoprecipitates only when cells are infected by both viruses (Fig. 3A Upper, lane 6). When cells are infected by a single virus, no TAFII170 protein can be detected (Fig. 3A Upper, lanes 4 and 5). Reprobing the blot with TBP antibodies indicated that immune complexes contain recombinant TBP as expected (Fig. 3A Lower, lanes 4–6). Also, recombinant TAFII170 and TBP were present in the lysates before immunoprecipitation (Fig. 3A, lanes 1–3).
Figure 3.
Recombinant TAFII170 and recombinant TBP associate after coexpression. (A) TAFII170 protein is precipitated by TBP antibodies only in the presence of recombinant TBP. A14 mouse fibroblast cells were infected with recombinant vaccinia viruses expressing TBP (lanes 1 and 4), TAFII170 (lanes 2 and 5), or TBP and TAFII170 (lanes 3 and 6) as indicated above the lanes. Cell lysates were subjected to immunoprecipitation by the TBP antibody 22D1. Immune complexes were washed and analyzed in lanes 4–6 on immunoblots with TBP or TAFII170 antibodies as indicated. Lanes 1–3 contain 20% of the input. Arrows and molecular size markers as in Fig. 1A. (B) Recombinant TBP coelutes with recombinant His-tagged TAFII170 during NiNTA affinity chromatography. Lysates of HeLa TK− cells infected with viruses expressing TBP (lanes 1–4), TAFII170 (lanes 5–8), or TBP and TAFII170 (lanes 9–12) were subjected to NiNTA–agarose chromatography. Fractions were analyzed on immunoblots using TAFII170 or TBP antibodies as indicated to the right. Load fractions were analyzed in lanes 1, 5, and 9; flow-through fractions in lanes 2, 6, and 10; wash fractions in lanes 3, 7, and 11; and elution fractions in lanes 4, 8, and 12. The asterisk indicates the heavy chain of the mouse 22D1 antibody.
To verify that recombinant TBP associates with TAFII170, another approach was required because the polyclonal TAFII170 antisera are not functional in immunoprecipitation. The presence of a His-tag in recombinant TAFII170 facilitates affinity purification. Lysates were prepared from infected HeLa cells and subjected to NiNTA affinity chromatography. Immunoblot analysis of the fractions showed that TBP is only present in the elution step of double-infected lysates (Fig. 3B Upper, lane 12). Part of the TBP is present in flow-through and wash fractions of the column (Fig. 3B Upper, lanes 2, 3, 10, and 11). This may indicate inefficient binding of recombinant TAFII170 to the column (Fig. 3B Lower, lanes 6 and 7) or that not all of the TBP is complexed with TAFII170. In the absence of recombinant TAFII170, TBP has a low affinity for NiNTA (Fig. 3A Upper, lane 3). In conclusion, these experiments show that recombinant TAFII170 can associate with recombinant TBP and verify that the isolated cDNA indeed encodes the TAF component of B-TFIID.
Analysis of (d)ATPase Activity of Recombinant TAFII170.
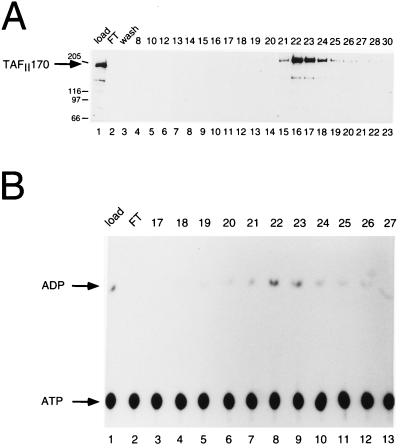
It has been shown that B-TFIID can hydrolyze both ATP and dATP (11). To test this for recombinant TAFII170, we purified the protein by a combination of ion exchange and affinity chromatography. After the NiNTA affinity step, no endogenous TBP was present in the TAFII170 fractions (data not shown and see also Fig. 3B Upper). Peak fractions were further purified and subsequently tested for presence of recombinant TAFII170 and for ATP hydrolysis. Fig. 4 A and B show that fractions 22 and 23 contain the TAFII170 peak and have the highest ATPase activity. Further analysis of fraction 23 indicated that the Km for ATP ranges between 30 and 50 μM (data not shown). The reaction is dependent on Mg ions and can utilize dATP. These biochemical properties of recombinant TAFII170 are very similar to those of B-TFIID (11). However, recent findings in the yeast system show stimulation of Mot1p’s ATPase activity by TBP (24). To investigate this for the human system, a direct comparison of TAFII170 and B-TFIID is required.
Figure 4.
Recombinant TAFII170 contains ATPase activity. (A) Immunoblot analysis of recombinant TAFII170. Fractions of the heparin affinity step were analyzed on immunoblots using TAFII170 antibodies as in Fig. 1B. Lane 1 contains 5 μl of the load, and lanes 2 and 3 contain 15 μl of the flow-through and wash fractions. Lanes 4–23 contain 3 μl of the fractions as indicated above. Arrows and molecular size markers are as in Fig. 1A. (B) ATPase activity assay of heparin fractions containing TAFII170 protein. Two microliters of the load (lane 1) or flow-through (lane 2) and 1 μl of heparin fraction 17–27 (lanes 3–13) were analyzed for ATPase activity. Positions of ATP and ADP are indicated by arrows.
DISCUSSION
In this paper, we describe the isolation of the cDNA for TAFII170. In a variety of assays, it is shown that the protein encoded by this cDNA is the TAF subunit of B-TFIID. The ability to hydrolyze (d)ATP is conferred to B-TFIID by the TAFII170 subunit as indicated by biochemical analysis of recombinant TAFII170. Of interest, the human TAFII170 gene has homologs in other organisms, the yeast MOT1 and Drosophila moira genes. These genes have been identified as global regulators of pol II transcription (21, 23) because mutations in these genes can affect transcription of a particular gene both positively and negatively. The relationship of TAFII170 with global regulators of pol II in other organisms strongly argues against previous suggestions that the TAF component of B-TFIID is involved in the pol III transcription system (12).
The yeast MOT1 gene was initially isolated as a repressor of basal pol II transcription by two independent screens (21, 28). Recent studies indicated that Mot1p can also stimulate pol II transcription from certain genes (27, 29, 30). The MOT1 gene is essential for yeast viability, which also is observed for other TAF genes (8, 9). These findings classify Mot1p as a global regulator of pol II transcription. A model for its biochemical action is provided by the finding of Hahn and coworkers (26) that Mot1p is identical to ADI (ATP-dependent inhibitor of TBP binding). Mot1p/ADI can remove TBP from the TATA box depending on ATP-hydrolysis, and this suggests that Mot1p redistributes TBP among different promoters. It was proposed that the increased mobility of TBP favors weak TATA boxes, explaining the stimulatory effect of Mot1p on TATA-less promoters (27, 30). In addition, part of the total Mot1p in yeast extracts exists in a complex with TBP (20), and it was shown very recently that the N-terminal 1281 amino acids of Mot1p are essential for TBP binding (24). In vitro transcription experiments analyzing repression by Leu3p indicated that Mot1p/TBP-containing fractions but not recombinant TBP support Leu3p repression (31). However, it is unclear if the other TAFs present in the assay are important for the corepression, and the molecular mechanism has not been determined yet. Preliminary experiments indicate that expression of human TAFII170 does not suppress mot1–1 or mot1–1033 mutant alleles (H.Th.M.T. and M. A. Collart, unpublished results).
The TAFII170 homolog in Drosophila melanogaster is the 89B helicase protein (22), which is encoded by the moira gene. Immunological analysis indicated that the gene encodes a protein of ≈210 kDa and showed a widespread expression during development. Of interest, in the latter part of embryogenesis, the protein accumulates in the ventral nerve cord and brain (22). Analysis of polytene chromosomes showed a distinct patterning, which indicates association with particular target genes (22). In analogy to TAFII170, it is likely that, in Drosophila cell extracts, the 89B helicase/moira protein is also present in a TBP–TAF complex and that TBP is responsible for its association with chromatin. Similar to MOT1, moira is essential for viability, and this strongly suggests that inactivation of both TAFII170 alleles in mice will be lethal. The moira gene was isolated as an activator of homeotic gene expression and belongs to the Trithorax group of genes (23). This heterogenous group also includes Drosophila brahma, which also belongs to the SNF2 family. Members of this family contain a DNA-stimulated (d)ATPase activity and are present in various chromatin-remodeling complexes. Destabilization of chromatin structures may explain the global activation properties of proteins like brahma (for review see ref. 19).
What are the implications of the Mot1p and moira results for TAFII170? It is probable that both activation and repression by Mot1p are caused by an increased redistribution of TBP–TAF complexes over pol II promoters (26). Supporting this model is the observation that B-TFIID rapidly repartitions over different promoters in template commitment assays (7). Temporally restricted genes like homeotic genes may be particularly sensitive to an impaired mobility of TBP, and this could explain the isolation of moira as a Trithorax gene. Alternatively, B-TFIID could act as a cellular reservoir for TBP. Transient overexpression of TBP in mammalian cells results in free TBP with a short half-life of 90 min whereas the average half-life of a TBP–TAF complex in the cells exceeds 12 h (F. C. P. Holstege and H.Th.M.T., unpublished observations). It is possible that TAFII170 performs a chaperone-like function for TBP. The reservoir model does not explain the classification of TAFII170 homologs as global pol II regulators. Because TBP is also essential for pol I and III transcription (1), one would expect effects of MOT1/moira mutations on these systems.
It is interesting to compare TAFII170 of B-TFIID with TAFII250 of the (D-)TFIID complex. Both proteins contain enzymatic activities. TAFII170 harbors a (d)ATPase activity, and TAFII250 has acetyltransferase and protein kinase activities (32, 33). Both can bind TBP in the absence of other proteins, resulting in reduced binding of TBP to DNA (7, 34). Two lines of evidence suggest that (D-)TFIID has gene specificity. Its TAFs interact with transcription regulators in an activation domain-specific manner (2) and TAF mutations can lead to specific cell cycle arrest phenotypes (35, 36). In contrast, the present evidence indicates that the B-TFIID complex functions in pol II transcription in a global manner.
In this discussion, we assumed that the functions of Mot1p, moira, and TAFII170 are dependent on interaction with TBP. It is important to stress that part of the cellular TAFII170 may not be complexed to TBP. In fact, size fractionation of yeast extracts showed that Mot1p is also present in complexes lacking TBP (20). During purification from HeLa cell extracts, we observed strict coelution of TAFII170 with TBP, but small amounts of TAFII170 protein may have escaped detection by our polyclonal antibodies. The availability of the TAFII170 cDNA clone allows development of better immunological reagents and facilitates further investigation of its role in the global regulation of pol II transcription in mammalian cells.
Acknowledgments
We thank the Bernards brothers for the gift of the B cell cDNA library and F. van Mansfeld for the mouse cDNA library. We also thank H. Stunnenberg for the recombinant TBP vaccinia virus and the pATA-gpt plasmid. We thank D. Reinberg for sharing unpublished results and M. Collart for help with the yeast experiments and for yeast strains. We acknowledge the contributions of R. Schiphof in providing HeLa cells and of F. Holstege in the isolation of TBP antibodies. We thank H. Stunnenberg and members of our lab for discussions. We also thank U. Fiedler and M. Walhout for critical reading of this manuscript. J. W. Borst was supported by the Dutch Cancer Society. This work was also supported by the Netherlands Foundation for Chemical Research.
ABBREVIATIONS
- TBP
TATA-binding protein
- TAF
TBP-associated factor
- pol
RNA polymerase
- TF
transcription factor
- β-ME
β-mercaptoethanol
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AJ001017).
References
- 1.Sharp P A. Cell. 1992;68:819–821. doi: 10.1016/0092-8674(92)90023-6. [DOI] [PubMed] [Google Scholar]
- 2.Burley S K, Roeder R G. Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 3.Jacq X, Brou C, Lutz Y, Davidson I, Chambon P, Tora L. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- 4.Chen J-L, Attardi L D, Verrijzer C P, Yokomori K, Tjian R. Cell. 1994;79:93–105. doi: 10.1016/0092-8674(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 5.Verrijzer C P, Chen J L, Yokomori K, Tjian R. Cell. 1995;81:1115–1125. doi: 10.1016/s0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotti A, Lutz Y, Heard D J, Chambon P, Tora L. EMBO J. 1996;15:5022–5031. [PMC free article] [PubMed] [Google Scholar]
- 7.Timmers H T M, Sharp P A. Genes Dev. 1991;5:1946–1956. doi: 10.1101/gad.5.11.1946. [DOI] [PubMed] [Google Scholar]
- 8.Moqtaderi Z, Bai Y, Poon D, Weil T. Nature (London) 1996;383:188–191. doi: 10.1038/383188a0. [DOI] [PubMed] [Google Scholar]
- 9.Walker S S, Reese J C, Apone L M, Green M R. Nature (London) 1996;383:185–188. doi: 10.1038/383185a0. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y J, Bjorklund S, Li Y, Sayre M H, Kornberg R D. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 11.Timmers H T M, Meyers R E, Sharp P A. Proc Natl Acad Sci USA. 1992;89:8140–8144. doi: 10.1073/pnas.89.17.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taggart A K P, Fisher T S, Pugh B F. Cell. 1992;71:1015–1028. doi: 10.1016/0092-8674(92)90396-t. [DOI] [PubMed] [Google Scholar]
- 13.Lobo S M, Tanaka M, Sullivan M L, Hernandez N. Cell. 1992;71:1029–1040. doi: 10.1016/0092-8674(92)90397-u. [DOI] [PubMed] [Google Scholar]
- 14.Teichmann M, Seifart K H. EMBO J. 1995;14:5974–5983. doi: 10.1002/j.1460-2075.1995.tb00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyers R E, Sharp P A. Mol Cell Biol. 1993;13:7953–7960. doi: 10.1128/mcb.13.12.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.Walhout A J M, Gubbels J M, Bernards R, van der Vliet P C, Timmers H T M. Nucleic Acids Res. 1997;25:1493–1501. doi: 10.1093/nar/25.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 19.Pazin M J, Kadonaga J T. Cell. 1997;88:737–740. doi: 10.1016/s0092-8674(00)81918-2. [DOI] [PubMed] [Google Scholar]
- 20.Poon D, Campbell A M, Bai Y, Weil P A. J Biol Chem. 1994;269:23135–23140. [PubMed] [Google Scholar]
- 21.Davis J L, Kunisawa R, Thorner J. Mol Cell Biol. 1992;12:1879–1892. doi: 10.1128/mcb.12.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman-Levi R, Miller C, Bogoch J, Zak N B. Nucleic Acids Res. 1996;24:3121–3128. doi: 10.1093/nar/24.16.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kennison J A, Tamkun J W. Proc Natl Acad Sci USA. 1988;85:8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Auble D T, Wang D, Post K W, Hahn S. Mol Cell Biol. 1997;17:4842–4851. doi: 10.1128/mcb.17.8.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auble D T, Hansen K E, Mueller C G F, Lane W S, Thorner J, Hahn S. Genes Dev. 1994;8:1920–1934. doi: 10.1101/gad.8.16.1920. [DOI] [PubMed] [Google Scholar]
- 27.Collart M A. Mol Cell Biol. 1996;16:6668–6676. doi: 10.1128/mcb.16.12.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piatti S, Tazzi R, Pizzagalli A, Plevani P, Lucchini G. Chromosoma. 1992;102:107–113. doi: 10.1007/BF02451793. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y W, Stillman D J. Genes Dev. 1996;10:604–619. doi: 10.1101/gad.10.5.604. [DOI] [PubMed] [Google Scholar]
- 30.Madison J M, Winston F. Mol Cell Biol. 1997;17:287–295. doi: 10.1128/mcb.17.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wade P A, Jaehning J A. Mol Cell Biol. 1996;16:1641–1648. doi: 10.1128/mcb.16.4.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizzen C A, Yang X J, Kokubo T, Brownell J E, Bannister A J, Owen Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 33.Dikstein R, Ruppert S, Tjian S. Cell. 1996;84:781–790. doi: 10.1016/s0092-8674(00)81055-7. [DOI] [PubMed] [Google Scholar]
- 34.Kokubo T, Gong D W, Yamashita S, Horikoshi M, Roeder R G, Nakatani Y. Genes Dev. 1993;7:1033–1046. doi: 10.1101/gad.7.6.1033. [DOI] [PubMed] [Google Scholar]
- 35.Wang E H, Tjian R. Science. 1994;263:811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- 36.Apone L M, Ching-man A V, Reese J C, Green M R. Genes Dev. 1996;10:2368–2380. doi: 10.1101/gad.10.18.2368. [DOI] [PubMed] [Google Scholar]