Abstract
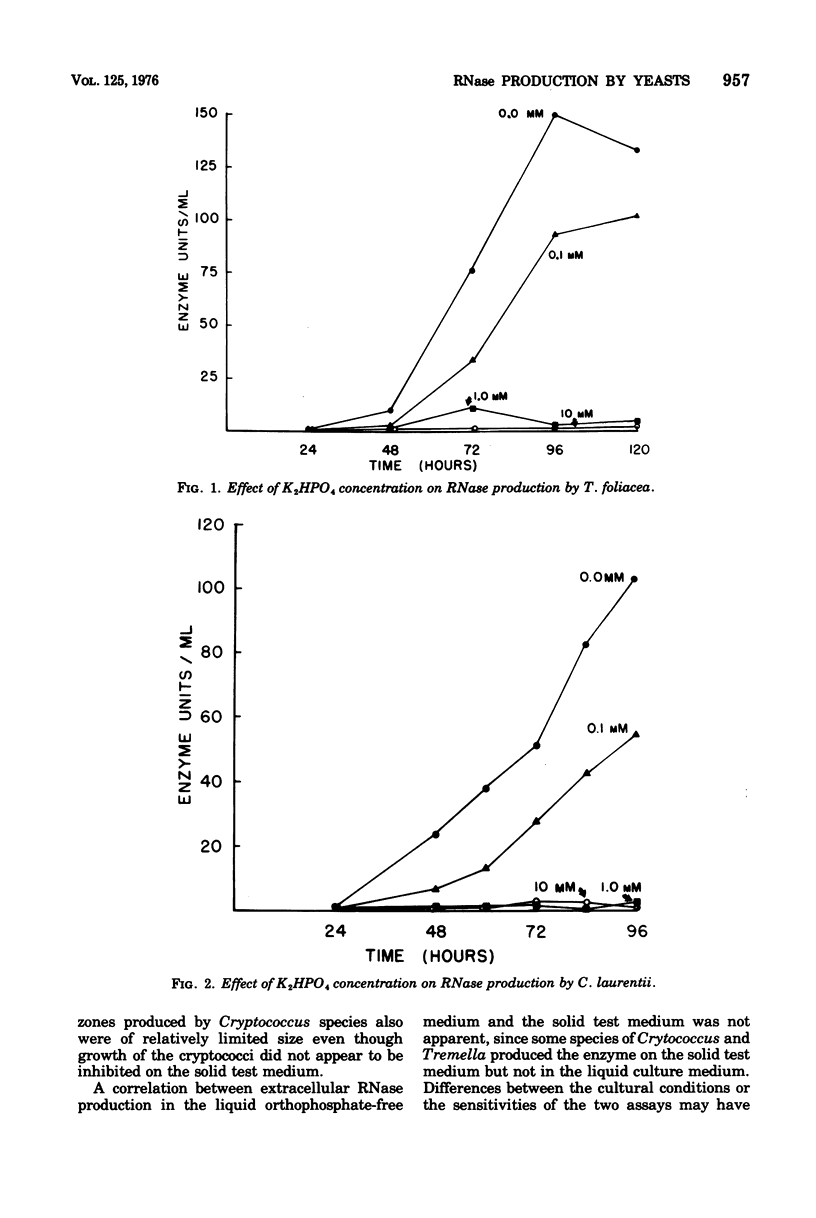
A strain of Cryptococcus laurentii and a haploid isolate of Tremella foliacea were shown to produce orthophosphate-repressible ribonuclease in liquid culture. Addition of as little as 1 mM K2HPO4, pH 7.0, completely repressed enzyme production by both fungi. The orthophosphate-repressible enzyme was not produced by other species of the two genera tested. These results, together with other findings, suggest a close phylogenetic relationship between Cryptococcus laurentii and Tremella foliacea. The ability of other yeasts and yeastlike fungi to hydrolyze ribonucleic acid in a solid test medium was assessed. Based on the limited number of organisms available for study, extracellular ribonuclease activity was found in species having close affinity to the Basidiomycetes and in yeasts classified in the ascomycetous genera, Endomycopsis, Hansenula, and Kluyveromyces. Other ascomycetous yeasts did not exhibit extracellular ribonuclease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blank A., Dekker C. A. Ribonuclease U 4 from Ustilago sphaerogena. Purification and physical properties. Biochemistry. 1972 Oct 10;11(21):3956–3962. doi: 10.1021/bi00771a019. [DOI] [PubMed] [Google Scholar]
- Cazin J., Jr, Kozel T. R., Lupan D. M., Burt W. R. Extracellular deoxyribonuclease production by yeasts. J Bacteriol. 1969 Nov;100(2):760–762. doi: 10.1128/jb.100.2.760-762.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell J. W., Statzell A. C., Hunter I. L., Phaff H. J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie Van Leeuwenhoek. 1969;35(4):433–462. [PubMed] [Google Scholar]
- GLITZ D. G., DEKKER C. A. STUDIES ON A RIBONUCLEASE FROM USTILAGO SPHAEROGENA. I. PURIFICATION AND PROPERTIES OF THE ENZYME. Biochemistry. 1964 Oct;3:1391–1399. doi: 10.1021/bi00898a001. [DOI] [PubMed] [Google Scholar]
- Hasunuma K. Repressible extracellular nucleases in Neurospora crassa. Biochim Biophys Acta. 1973 Sep 7;319(3):288–293. doi: 10.1016/0005-2787(73)90168-8. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. M., Nyc J. F., Brown D. M. Isolation and chemical properties of a repressible acid phosphatase in Neurospora crassa. J Biol Chem. 1971 Mar 10;246(5):1419–1425. [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman C. P. Formation of hyphae and chlamydospores by Cryptococcus laurentii. Mycologia. 1973 Mar-Apr;65(2):388–395. [PubMed] [Google Scholar]
- Newell S. Y., Hunter I. L. Rhodosporidium diobovatum sp. n., the perfect form of an asporogenous yeast (Rhodotorula sp.). J Bacteriol. 1970 Oct;104(1):503–508. doi: 10.1128/jb.104.1.503-508.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues de Miranda L. Filobasidum capsuligenum nov. comb. Antonie Van Leeuwenhoek. 1972;38(1):91–99. doi: 10.1007/BF02328080. [DOI] [PubMed] [Google Scholar]
- Slodki M. E., Wickerham L. J., Bandoni R. J. Extracellular heteropolysaccharides from Cryptococcus and Tremella. A possible taxonomic relationship. Can J Microbiol. 1966 Jun;12(3):489–494. doi: 10.1139/m66-071. [DOI] [PubMed] [Google Scholar]
- Storck R., Alexopoulos C. J. Deoxyribonucleic acid of fungi. Bacteriol Rev. 1970 Jun;34(2):126–154. doi: 10.1128/br.34.2.126-154.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storck R., Alexopoulos C. J., Phaff H. J. Nucleotide composition of deoxyribonucleic acid of some species of Cryptococcus, Rhodotorula, and Sporobolomyces. J Bacteriol. 1969 Jun;98(3):1069–1072. doi: 10.1128/jb.98.3.1069-1072.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Walt J. P. The perfect and imperfect states of Sporobolomyces salmonicolor. Antonie Van Leeuwenhoek. 1970;36(1):49–55. doi: 10.1007/BF02069007. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., ORTON W. L. REPRESSIBLE ACID PHOSPHOMONOESTERASE AND CONSTITUTIVE PYROPHOSPHATASE OF SACCHAROMYCES MELLIS. J Bacteriol. 1963 Oct;86:805–813. doi: 10.1128/jb.86.4.805-813.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Walt J. P. Aessosporon dendrophilum sp. nov., the perfect state of Bullera dendrophila. Antonie Van Leeuwenhoek. 1973;39(3):455–460. doi: 10.1007/BF02578888. [DOI] [PubMed] [Google Scholar]


