Abstract
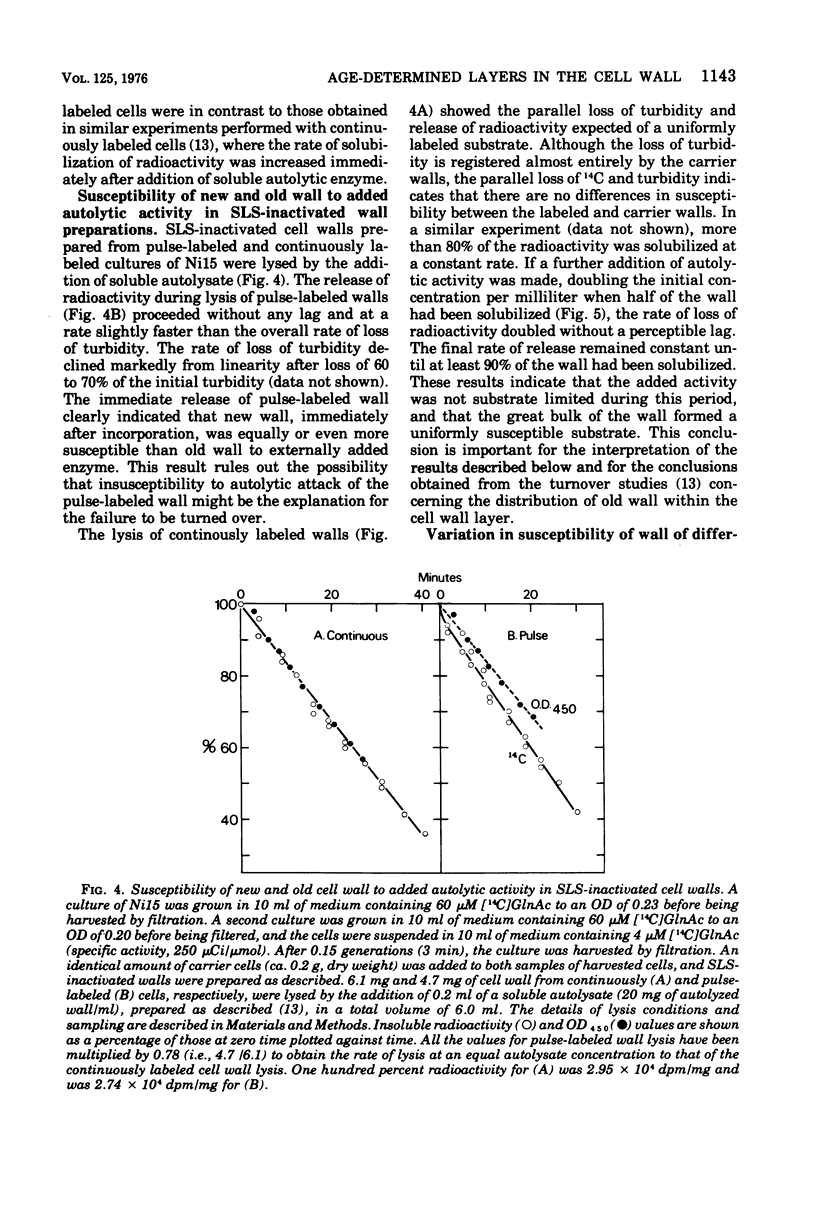
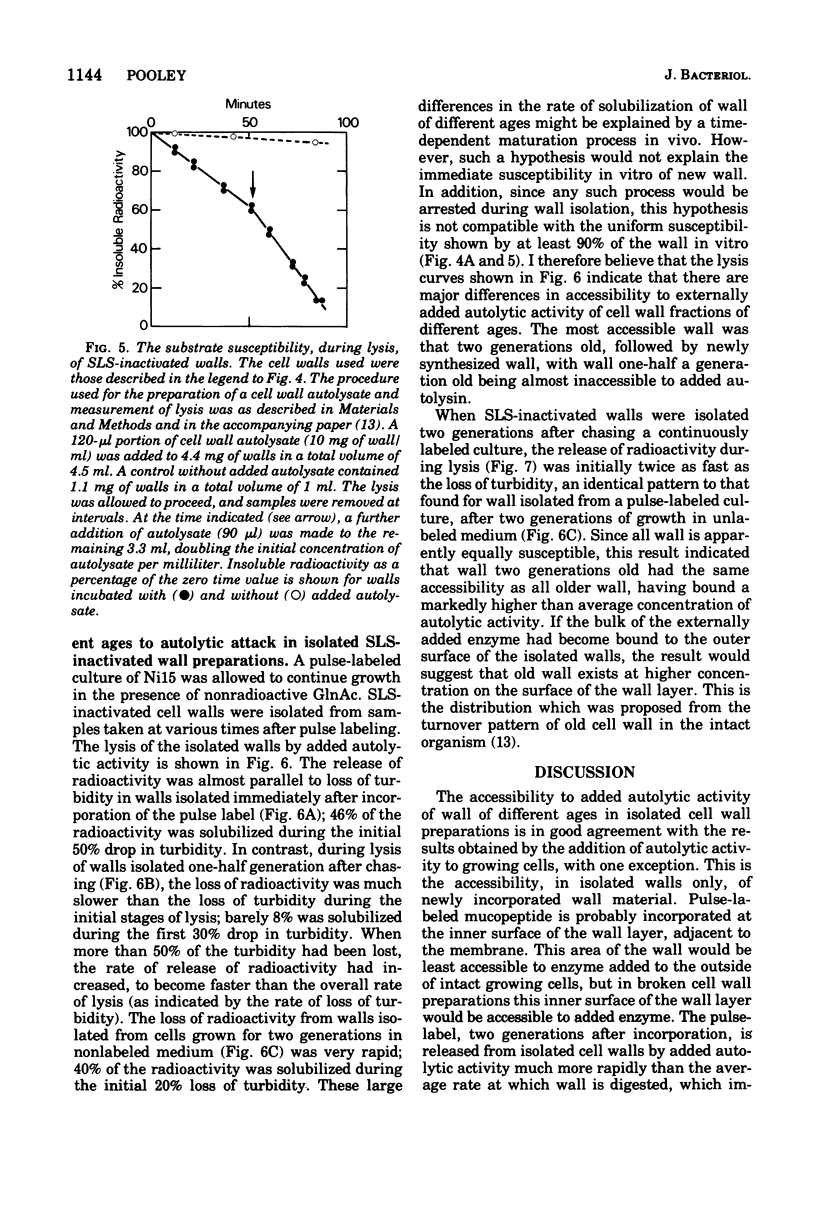
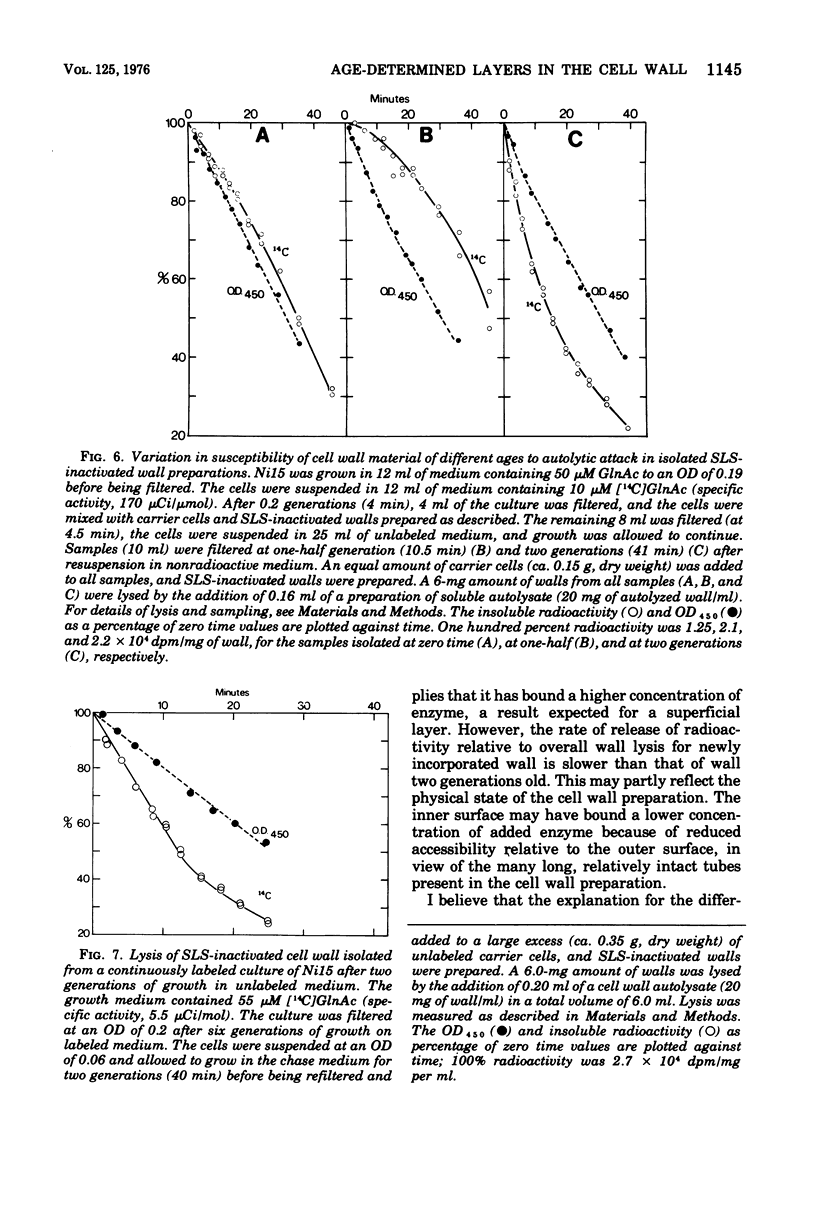
When soluble autolytic activity was added to growing cultures of a mutant possessing a reduced rate of cell wall turnover, there was a delay of more than one generation before solubilization of new cell wall began, in contrast to the immediate increase in the rate of solubilization of old cell wall. A similar delay was found before turnover of new cell wall occurred in the parent, in agreement with a previous report (Mauck et al., 1971). When sodium lauryl sulfate-inactivated cell walls were prepared, the great bulk of the wall formed a uniformly susceptible substrate to added autolytic activity. The immediate solubilization of new wall eliminates insusceptibility to autolytic enzyme as an explanation for the failure to be turned over. There were, however, major differences in the rate of solubilization of wall of different ages. During solubilization of the initial 30% of the cell wall preparation, wall two generations old was solubilized at least seven times faster than wall one-half a generation old. This result is interpreted in terms of differences in accessibility. The cell wall is seen as consisting of a series of layers, the age of which increases with the distance from the membrane, such that wall newly synthesized on the membrane passes out through the thickness of the cell wall layer during subsequent growth and only becomes susceptible to turnover as it reaches the outer surface, largely in the form of a layer, more than one generation after incorporation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boothby D., Daneo-Moore L., Higgins M. L., Coyette J., Shockman G. D. Turnover of bacterial cell wall peptidoglycans. J Biol Chem. 1973 Mar 25;248(6):2161–2169. [PubMed] [Google Scholar]
- Briles E. B., Tomasz A. Radioautographic evidence for equatorial wall growth in a gram-positive bacterium. Segregation of choline-3H-labeled teichoic acid. J Cell Biol. 1970 Dec;47(3):786–790. doi: 10.1083/jcb.47.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG K. L., HAWIRKO R. Z., ISAAC P. K. CELL WALL REPLICATION. I. CELL WALL GROWTH OF BACILLUS CEREUS AND BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:43–48. doi: 10.1139/m64-007. [DOI] [PubMed] [Google Scholar]
- Chaloupka J. Synthesis and degradation of surface structures by growing and non-growing Bacillus megaterium. Folia Microbiol (Praha) 1967;12(3):264–273. doi: 10.1007/BF02868742. [DOI] [PubMed] [Google Scholar]
- Chung K. L. Autoradiographic studies of bacterial cell wall replication. I. Cell wall growth of Bacillus cereus in the presence of chloramphenicol. Can J Microbiol. 1967 Apr;13(4):341–350. doi: 10.1139/m67-046. [DOI] [PubMed] [Google Scholar]
- Cole R. M. Symposium on the fine structure and replication of bacteria and their parts. 3. Bacterial cell-wall replication followed by immunofluorescence. Bacteriol Rev. 1965 Sep;29(3):326–344. doi: 10.1128/br.29.3.326-344.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frehel C., Beaufils A. M., Ryter A. Etude au microscope électronique de la croissance de la paroi chez B. subtilis et B. megaterium. Ann Inst Pasteur (Paris) 1971 Aug;121(2):139–148. [PubMed] [Google Scholar]
- Hughes R. C., Stokes E. Cell wall growth in Bacillus licheniformis followed by immunofluorescence with mucopeptide-specific antiserum. J Bacteriol. 1971 May;106(2):694–696. doi: 10.1128/jb.106.2.694-696.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCZURA E., PERKINS H. R., ROGERS H. J. Teichuronic acid: a mucopolysaccharide present in wall preparations from vegetative cells of Bacillus subtilis. Biochem J. 1961 Jul;80:82–93. doi: 10.1042/bj0800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C., Hirota Y., Jacob F. On the process of cellular division in Escherichia coli. VI. Use of a methocel-autoradiographic method for the study of cellular division in Escherichia coli. J Bacteriol. 1971 Oct;108(1):375–385. doi: 10.1128/jb.108.1.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L. Turnover of the cell wall of Gram-positive bacteria. J Biol Chem. 1971 Mar 25;246(6):1820–1827. [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L., Williamson J. Mode of cell wall growth of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):373–378. doi: 10.1128/jb.109.1.373-378.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M. Turnover and spreading of old wall during surface growth of Bacillus subtilis. J Bacteriol. 1976 Mar;125(3):1127–1138. doi: 10.1128/jb.125.3.1127-1138.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E. Variation in the chemical composition of the cell walls of Bacillus subtilis during growth in different media. Nature. 1965 Jul 3;207(992):104–105. doi: 10.1038/207104b0. [DOI] [PubMed] [Google Scholar]


