Abstract
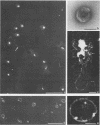
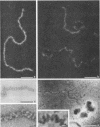
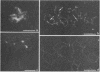
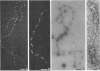
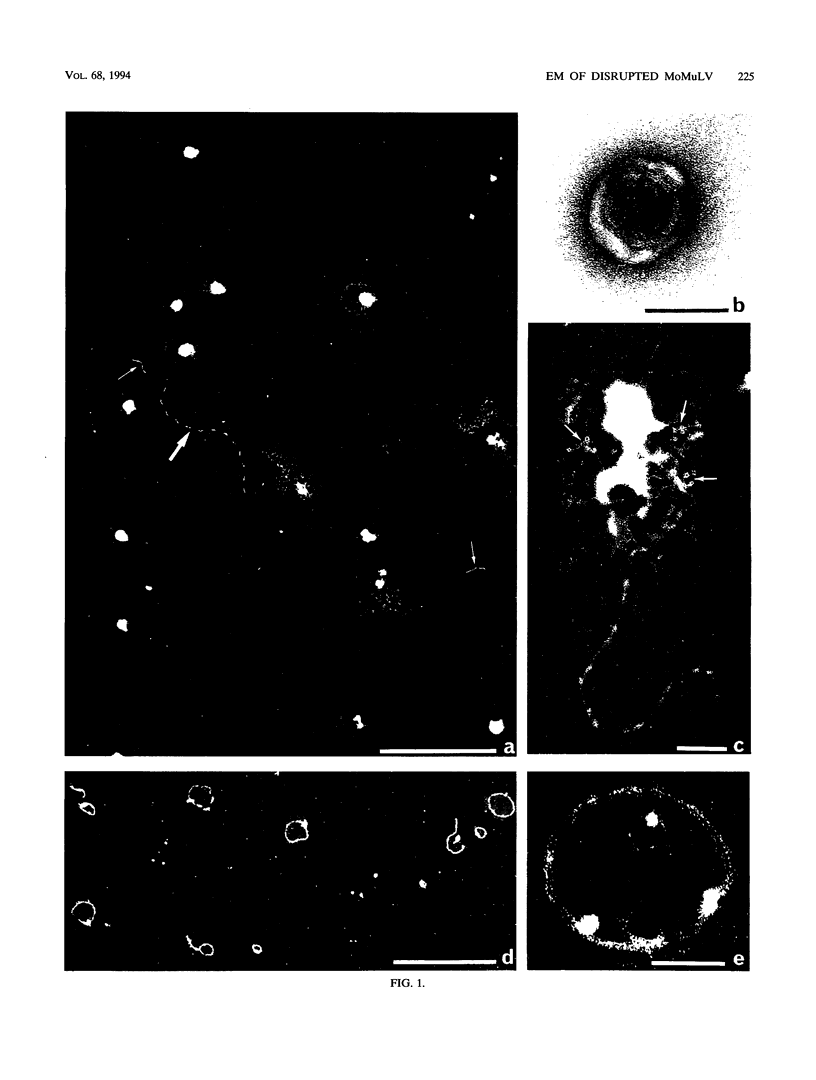
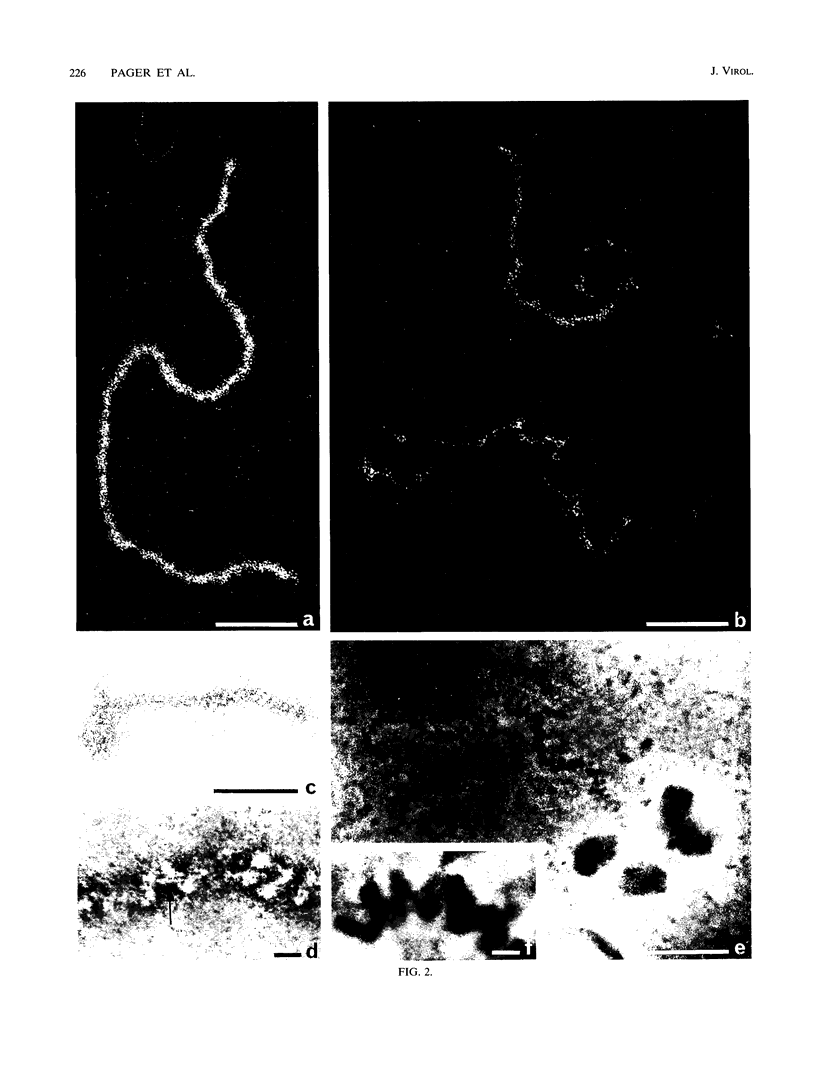
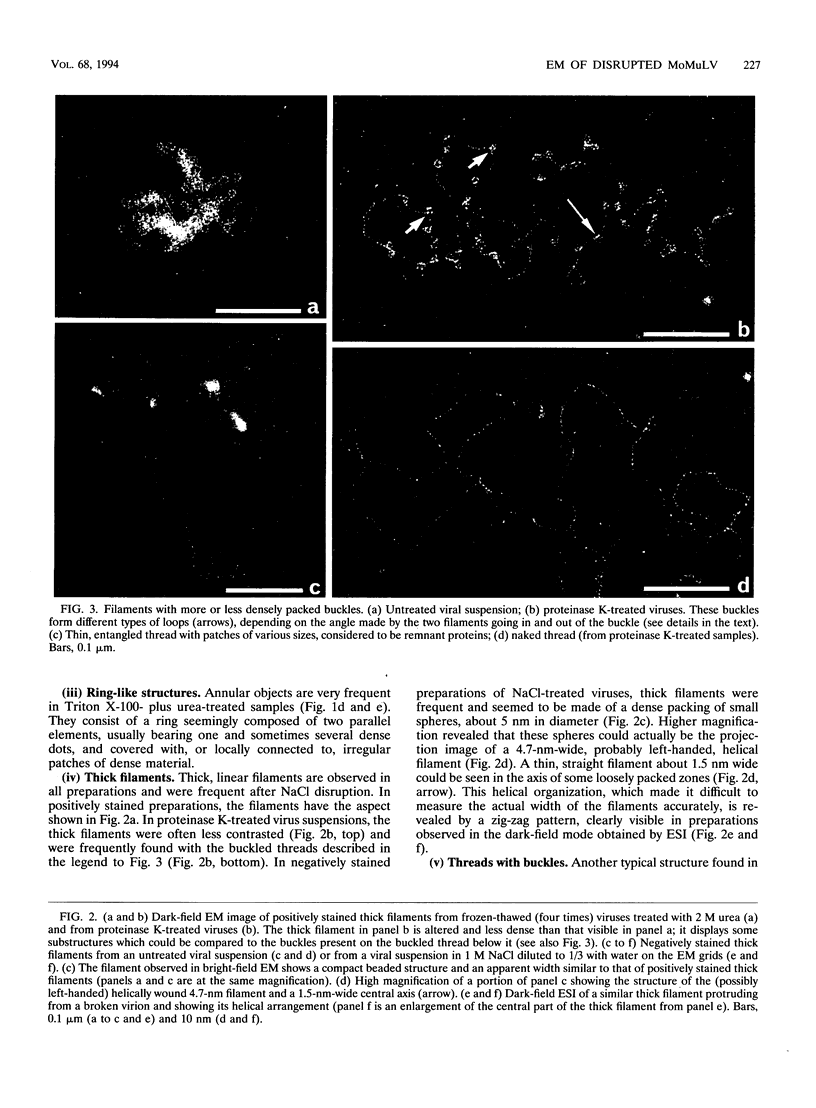
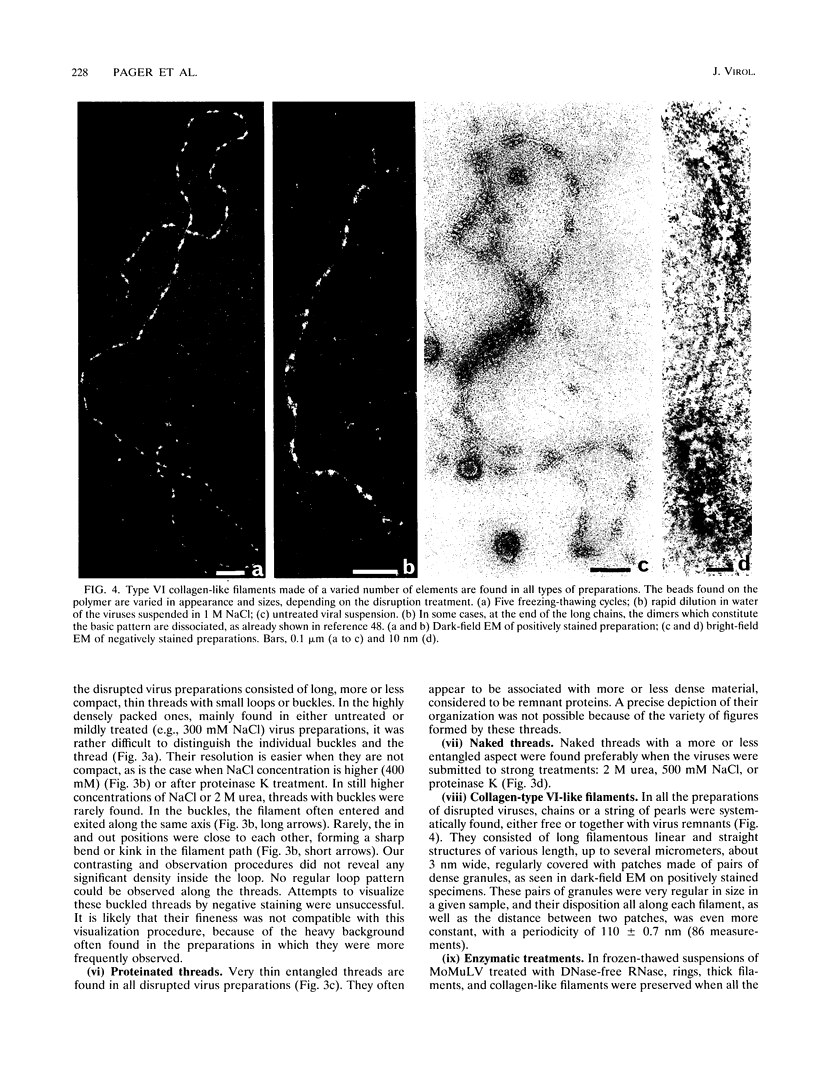
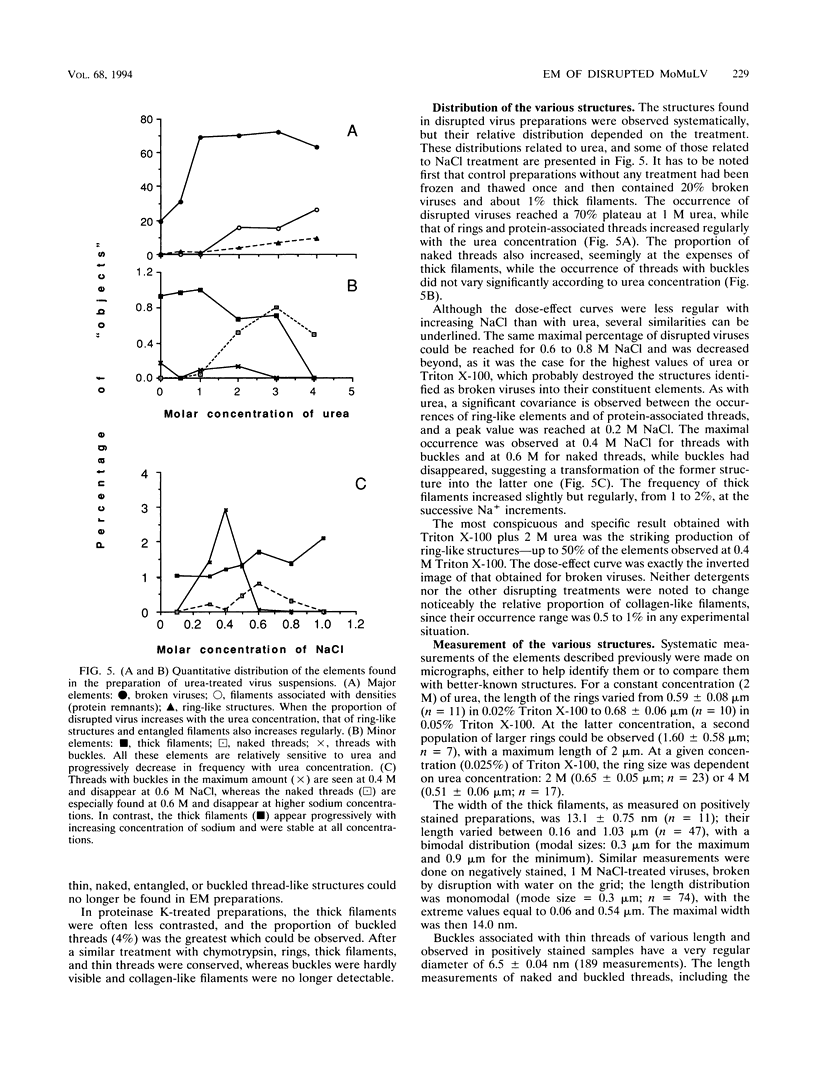
To analyze the constituents of retroviruses, the Moloney murine leukemia virus was disrupted and observed by dark-field electron microscopy. Virus disruption was achieved by several methods: osmotic shock, freezing-thawing cycles, and exposure to urea up to 4 M, to NaCl up to 1 M, and to Triton X-100. Several components associated with broken Moloney murine leukemia virus were repeatedly found in preparations. These components have been described as rings, thick filaments, chain-like filaments, threads covered with proteins, threads with buckles, and naked threads. A quantitative analysis of the occurrence of these components has been carried out. Among them, the thick filaments composed of a compact helical arrangement of small beads 5 nm in diameter were considered to represent the nucleocapsid. The protease-sensitive buckles found on some threads could be a compact form of the viral RNA associated to the nucleocapsid protein NCp10. The RNase-sensitive naked threads are interpreted as the deproteinized viral RNA itself. The ubiquitous chain-like filaments possess a periodic structure identical to that of polymerized type VI collagen. It is proposed that this adhesive protein is associated with the viral envelope taken from the cell membrane during the budding process of retroviruses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Barbieri D., Delain E., Lazar P., Hue G., Barski G. Method of virus particle counting using millipore filtration. Virology. 1970 Oct;42(2):544–547. doi: 10.1016/0042-6822(70)90299-0. [DOI] [PubMed] [Google Scholar]
- Bender W., Chien Y. H., Chattopadhyay S., Vogt P. K., Gardner M. B., Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978 Mar;25(3):888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P., Montelaro R. C., Sullivan S. J. A model for assembly of type-c oncornaviruses. Med Microbiol Immunol. 1977;164(1-3):97–113. doi: 10.1007/BF02121306. [DOI] [PubMed] [Google Scholar]
- Bruns R. R. Beaded filaments and long-spacing fibrils: relation to type VI collagen. J Ultrastruct Res. 1984 Nov;89(2):136–145. doi: 10.1016/s0022-5320(84)80010-6. [DOI] [PubMed] [Google Scholar]
- Chen M., Garon C. F., Papas T. S. Native ribonucleoprotein is an efficient transcriptional complex of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1296–1300. doi: 10.1073/pnas.77.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I. M., Yaniv A., Dahlberg J. E., Gazit A., Skuntz S. F., Tronick S. R., Aaronson S. A. Nucleotide sequence evidence for relationship of AIDS retrovirus to lentiviruses. 1985 Sep 26-Oct 2Nature. 317(6035):366–368. doi: 10.1038/317366a0. [DOI] [PubMed] [Google Scholar]
- De Rocquigny H., Ficheux D., Gabus C., Allain B., Fournie-Zaluski M. C., Darlix J. L., Roques B. P. Two short basic sequences surrounding the zinc finger of nucleocapsid protein NCp10 of Moloney murine leukemia virus are critical for RNA annealing activity. Nucleic Acids Res. 1993 Feb 25;21(4):823–829. doi: 10.1093/nar/21.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Burdsall A. L., Banerjee A. K. Role of cellular actin in human parainfluenza virus type 3 genome transcription. J Biol Chem. 1993 Mar 15;268(8):5703–5710. [PubMed] [Google Scholar]
- Delius H., Duesberg P. H., Mangel W. F. Electron microscope measurements of rous sarcoma virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):835–843. doi: 10.1101/sqb.1974.039.01.097. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Engel J., Furthmayr H., Odermatt E., von der Mark H., Aumailley M., Fleischmajer R., Timpl R. Structure and macromolecular organization of type VI collagen. Ann N Y Acad Sci. 1985;460:25–37. doi: 10.1111/j.1749-6632.1985.tb51154.x. [DOI] [PubMed] [Google Scholar]
- Feller U., Dougherty R. M., Di Stefano H. S. Comparative morphology of avian and murine leukemia viruses. J Natl Cancer Inst. 1971 Dec;47(6):1289–1298. [PubMed] [Google Scholar]
- Fowler W. E., Fretto L. J., Hamilton K. K., Erickson H. P., McKee P. A. Substructure of human von Willebrand factor. J Clin Invest. 1985 Oct;76(4):1491–1500. doi: 10.1172/JCI112129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank H., Schwarz H., Graf T., Schäfer W. Properties of mouse leukemia viruses. XV. Electron microscopic studies on the organization of Friend leukemia virus and other mammalian C-type viruses. Z Naturforsch C. 1978 Jan-Feb;33(1-2):124–138. doi: 10.1515/znc-1978-1-224. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991 Jun;5(6):617–637. [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom H., Bauer H., Ogura H., Wigand R., Fischer A. B. Detection of oncornavirus-like particles in HeLa cells. I. Fine structure and comparative morphological classification. Int J Cancer. 1974 Feb 15;13(2):246–253. doi: 10.1002/ijc.2910130212. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Bellard M., Oudet P., Chambon P. Stability of nucleosomes in native and reconstituted chromatins. Nucleic Acids Res. 1976 Nov;3(11):3173–3192. doi: 10.1093/nar/3.11.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Ikuta K., Zhang J. J., Morita C., Sano K., Komatsu M., Fujita H., Kato S., Nakai M. The budding of defective human immunodeficiency virus type 1 (HIV-1) particles from cell clones persistently infected with HIV-1. Arch Virol. 1990;111(1-2):87–101. doi: 10.1007/BF01310507. [DOI] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglund S., Ofverstedt L. G., Nilsson A., Lundquist P., Gelderblom H., Ozel M., Skoglund U. Spatial visualization of the maturing HIV-1 core and its linkage to the envelope. AIDS Res Hum Retroviruses. 1992 Jan;8(1):1–7. doi: 10.1089/aid.1992.8.1. [DOI] [PubMed] [Google Scholar]
- Katsumoto T., Hattori N., Kurimura T. Maturation of human immunodeficiency virus, strain LAV, in vitro. Intervirology. 1987;27(3):148–153. doi: 10.1159/000149733. [DOI] [PubMed] [Google Scholar]
- Kielty C. M., Whittaker S. P., Grant M. E., Shuttleworth C. A. Type VI collagen microfibrils: evidence for a structural association with hyaluronan. J Cell Biol. 1992 Aug;118(4):979–990. doi: 10.1083/jcb.118.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacour F., Fourcade A., Verger C., Delain E. Coiled structure of the nucleocapsid of avian myeloblastosis virus. J Gen Virol. 1970 Oct;9(1):89–92. doi: 10.1099/0022-1317-9-1-89. [DOI] [PubMed] [Google Scholar]
- Le Cam E., Théveny B., Mignotte B., Révet B., Delain E. Quantitative electron microscopic analysis of DNA-protein interactions. J Electron Microsc Tech. 1991 Aug;18(4):375–386. doi: 10.1002/jemt.1060180406. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Kilham S. S. An electron microscope study of Rauscher leukemia virus. Virology. 1971 Nov;46(2):277–297. doi: 10.1016/0042-6822(71)90030-4. [DOI] [PubMed] [Google Scholar]
- Maurer B., Flügel R. M. Genomic organization of the human spumaretrovirus and its relatedness to AIDS and other retroviruses. AIDS Res Hum Retroviruses. 1988 Dec;4(6):467–473. doi: 10.1089/aid.1988.4.467. [DOI] [PubMed] [Google Scholar]
- Murti K. G., Bondurant M., Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981 Jan;37(1):411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave D. R., Sandman K. M., Reeve J. N. DNA binding by the archaeal histone HMf results in positive supercoiling. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10397–10401. doi: 10.1073/pnas.88.23.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Senior M. B., Olins D. E. Ultrastructural features of chromatin nu bodies. J Cell Biol. 1976 Mar;68(3):787–793. doi: 10.1083/jcb.68.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon F., Favaudon V., Tourbez-Perrin M., Bieth J. Localization of the two protease binding sites in human alpha 2-macroglobulin. J Biol Chem. 1981 Jan 25;256(2):547–550. [PubMed] [Google Scholar]
- Pons M. W., Schulze I. T., Hirst G. K., Hauser R. Isolation and characterization of the ribonucleoprotein of influenza virus. Virology. 1969 Oct;39(2):250–259. doi: 10.1016/0042-6822(69)90045-2. [DOI] [PubMed] [Google Scholar]
- Revet B., Delain E. The drosophila X virus contains a 1-microM double-stranded RNA circularized by a 67-kd terminal protein: high-resolution denaturation mapping of its genome. Virology. 1982 Nov;123(1):29–44. doi: 10.1016/0042-6822(82)90292-6. [DOI] [PubMed] [Google Scholar]
- Rhodes D. Structural analysis of a triple complex between the histone octamer, a Xenopus gene for 5S RNA and transcription factor IIIA. EMBO J. 1985 Dec 16;4(13A):3473–3482. doi: 10.1002/j.1460-2075.1985.tb04106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar N. H., Nowinski R. C., Moore D. H. Helical nucleocapsid structure of the oncogenic ribonucleic acid viruses (oncornaviruses). J Virol. 1971 Oct;8(4):564–572. doi: 10.1128/jvi.8.4.564-572.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy D. G., Stein D. B. Nucleoprotein subunit structure in an unusual prokaryotic organism: Thermoplasma acidophilum. Biochim Biophys Acta. 1980 Aug 26;609(1):180–195. doi: 10.1016/0005-2787(80)90211-7. [DOI] [PubMed] [Google Scholar]
- Shioda M., Sugimori K., Shiroya T., Takayanagi S. Nucleosomelike structures associated with chromosomes of the archaebacterium Halobacterium salinarium. J Bacteriol. 1989 Aug;171(8):4514–4517. doi: 10.1128/jb.171.8.4514-4517.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Guerrero H., Peattie D. A., Meza I. Chromatin organization in Entamoeba histolytica. Mol Biochem Parasitol. 1991 Mar;45(1):121–130. doi: 10.1016/0166-6851(91)90033-3. [DOI] [PubMed] [Google Scholar]
- Touchette N. A., Cole R. D. Effects of salt concentration and H1 histone removal on the differential scanning calorimetry of nuclei. Biochemistry. 1992 Feb 18;31(6):1842–1849. doi: 10.1021/bi00121a037. [DOI] [PubMed] [Google Scholar]
- Weeks B. S., Desai K., Loewenstein P. M., Klotman M. E., Klotman P. E., Green M., Kleinman H. K. Identification of a novel cell attachment domain in the HIV-1 Tat protein and its 90-kDa cell surface binding protein. J Biol Chem. 1993 Mar 5;268(7):5279–5284. [PubMed] [Google Scholar]
- Woodcock C. L., Woodcock H., Horowitz R. A. Ultrastructure of chromatin. I. Negative staining of isolated fibers. J Cell Sci. 1991 May;99(Pt 1):99–106. doi: 10.1242/jcs.99.1.99. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Murcia G., Jongstra-Bilen J., Ittel M. E., Mandel P., Delain E. Poly(ADP-ribose) polymerase auto-modification and interaction with DNA: electron microscopic visualization. EMBO J. 1983;2(4):543–548. doi: 10.1002/j.1460-2075.1983.tb01460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rest M., Garrone R. Collagen family of proteins. FASEB J. 1991 Oct;5(13):2814–2823. [PubMed] [Google Scholar]
- von der Mark H., Aumailley M., Wick G., Fleischmajer R., Timpl R. Immunochemistry, genuine size and tissue localization of collagen VI. Eur J Biochem. 1984 Aug 1;142(3):493–502. doi: 10.1111/j.1432-1033.1984.tb08313.x. [DOI] [PubMed] [Google Scholar]