Abstract
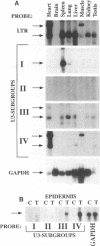
The murine VL30 elements constitute one family of retrotransposons represented in 100 to 200 copies that are dispersed among the mouse chromosomes. On the basis of sequence homology, we have subdivided mouse VL30 members into four distinct U3 subgroups. The use of subgroup-specific probes in Northern (RNA) blot analyses shows that individual VL30 U3 subgroups are expressed in a tissue-specific manner. We show by in situ hybridization of mouse skin treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) that VL30 expression is induced in epidermal keratinocytes but not in dermal fibroblasts. Transient transfections of reporter gene plasmids together with in vitro binding analysis indicate that TPA-induced VL30 transcription specific for keratinocytes is mediated by two cooperating sequence motifs in juxtaposed position. One sequence motif is shown to constitutively bind CREB- and Jun-related proteins in both keratinocytes and fibroblasts, whereas the other is a target for TPA-induced c-Rel/p65(NF-kappa B)-binding activity specifically in keratinocytes. These binding sites are found to be conserved within U3 subgroups and individual U3 regions showing induced expression in TPA-treated mouse epidermis. These results together with a sequence comparison between different U3 subgroups indicate that cell type-specific activity of transcription factors known to regulate VL30 transcription and the presence or absence of their cognate binding sites within individual U3 regions determine inducible and cell type-specific VL30 expression. The variable VL30 U3 regions might thus be useful tools to study inducible and cell type-specific transcription in many different cell systems.
Full text
PDF



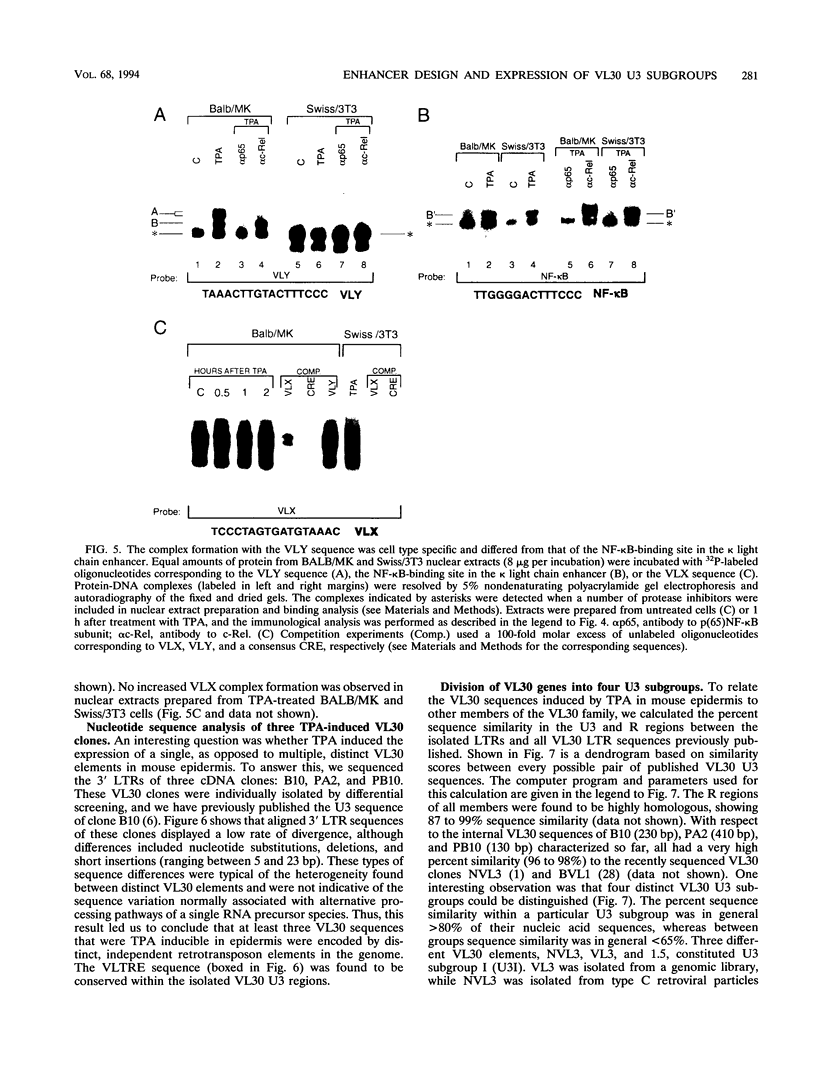
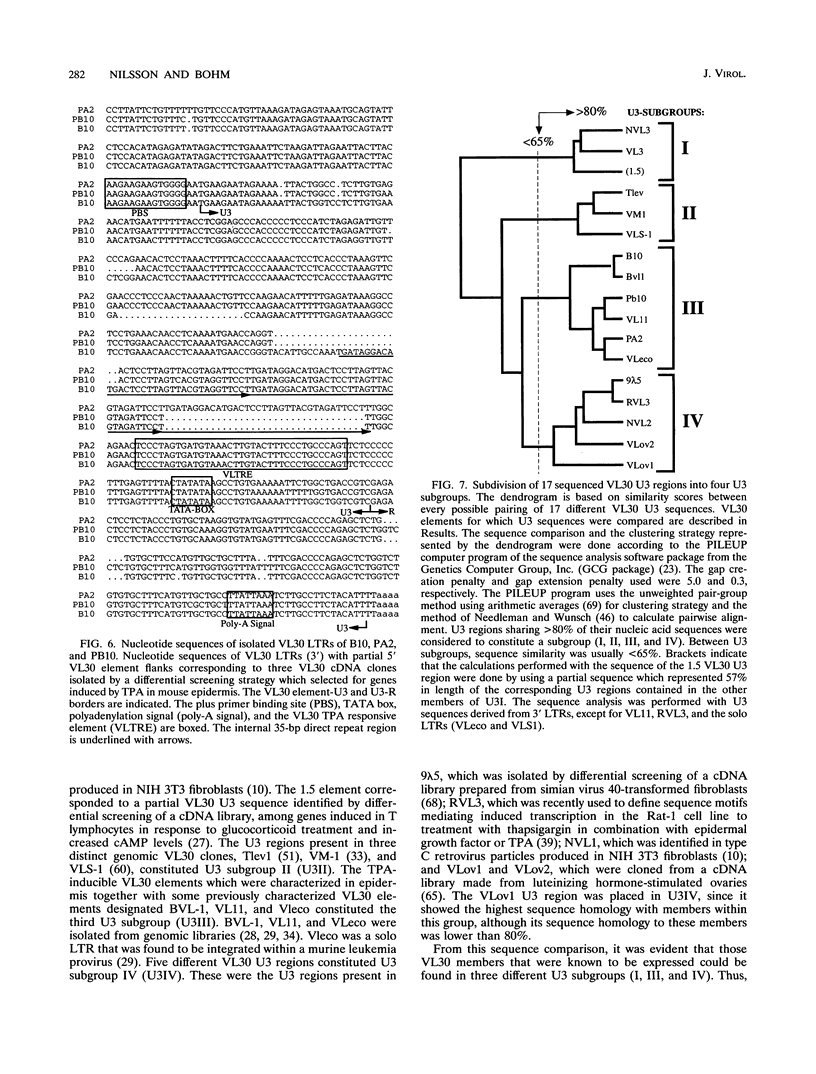

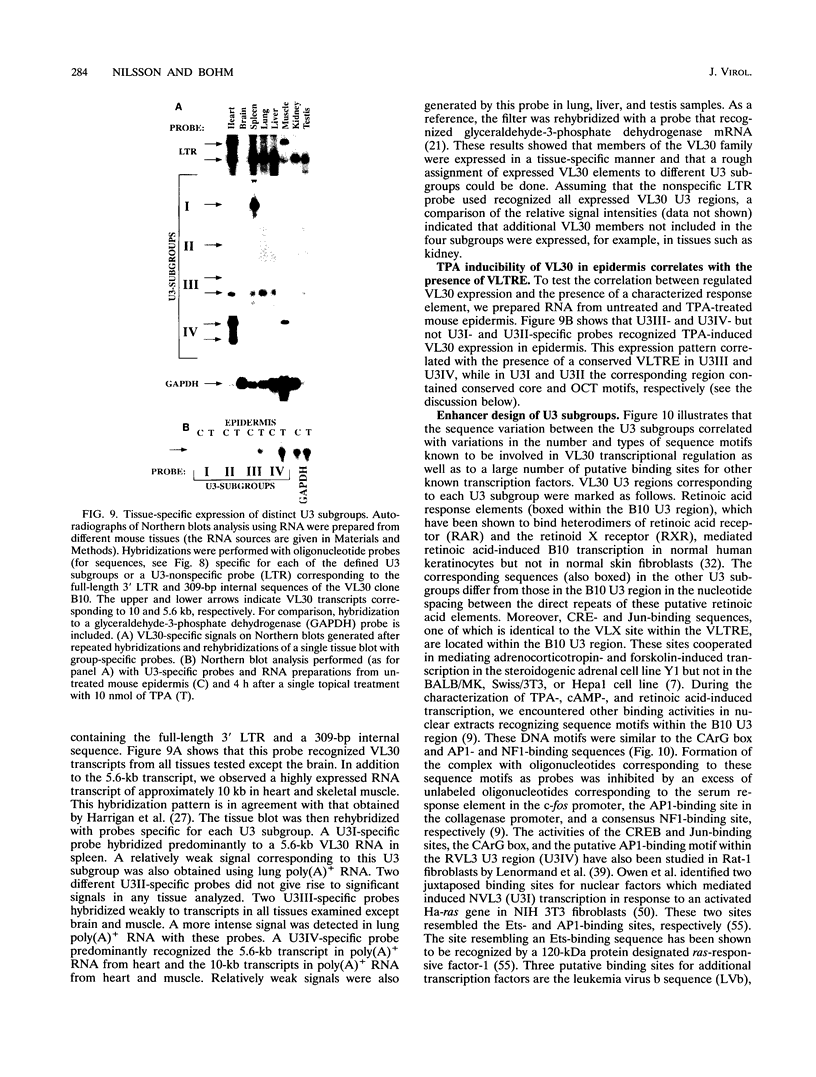




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Rathjen P. D., Stanway C. A., Fulton S. M., Malim M. H., Wilson W., Ogden J., King L., Kingsman S. M., Kingsman A. J. Complete nucleotide sequence of a mouse VL30 retro-element. Mol Cell Biol. 1988 Aug;8(8):2989–2998. doi: 10.1128/mcb.8.8.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz C. M., Conti C. J., Gimenez I. B., Slaga T. J., Klein-Szanto A. J. Cutaneous changes during prolonged application of 12-O-tetradecanoylphorbol-13-acetate on mouse skin and residual effects after cessation of treatment. Cancer Res. 1985 Jun;45(6):2753–2759. [PubMed] [Google Scholar]
- Anderson G. R., Stoler D. L., Scarcello L. A. Retrotransposon-like VL30 elements are efficiently induced in anoxic rat fibroblasts. J Mol Biol. 1989 Feb 20;205(4):765–769. doi: 10.1016/0022-2836(89)90320-3. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Dixon E. P., Peffer N. J., Bogerd H., Doerre S., Stein B., Greene W. C. The 65-kDa subunit of human NF-kappa B functions as a potent transcriptional activator and a target for v-Rel-mediated repression. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1875–1879. doi: 10.1073/pnas.89.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Corces V. G. Transcription and reverse transcription of retrotransposons. Annu Rev Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- Bohm S., Bakke M., Nilsson M., Zanger U. M., Spyrou G., Lund J. Cooperating nonconsensus cAMP-responsive elements are mediators of adrenocorticotropin-induced VL30 transcription in steroidogenic adrenal cells. J Biol Chem. 1993 Feb 25;268(6):3952–3963. [PubMed] [Google Scholar]
- Bohm S., Berghard A., Pereswetoff-Morath C., Toftgård R. Isolation and characterization of complementary DNA clones corresponding to genes induced in mouse epidermis in vivo by tumor promoters. Cancer Res. 1990 Mar 1;50(5):1626–1633. [PubMed] [Google Scholar]
- Bohm S. Identification of protein-binding sequences mediating constitutive and 12-O-tetradecanoylphorbol-13-acetate-induced VL30 transcription in cultured mouse and human keratinocytes. J Biol Chem. 1991 Dec 25;266(36):24834–24841. [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Avery R. J. A novel approach to cloning transcriptionally active retrovirus-like genetic elements from mouse cells. Nucleic Acids Res. 1983 Sep 24;11(18):6243–6254. doi: 10.1093/nar/11.18.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A. T., Norton J. D., Avery R. J. The genomic DNA organisation and evolution of a retrovirus-transmissible family of mouse (VL30) genetic elements. Biochim Biophys Acta. 1988 Nov 10;951(1):130–138. doi: 10.1016/0167-4781(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Courtney M. G., Elder P. K., Steffen D. L., Getz M. J. Evidence for an early evolutionary origin and locus polymorphism of mouse VL30 DNA sequences. J Virol. 1982 Aug;43(2):511–518. doi: 10.1128/jvi.43.2.511-518.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H. Y., Etzerodt M., Baekgaard A. J., Lovmand S., Jørgensen P., Kjeldgaard N. O., Pedersen F. S. Multiple sequence elements in the U3 region of the leukemogenic murine retrovirus SL3-2 contribute to cell-dependent gene expression. Virology. 1990 Apr;175(2):581–585. doi: 10.1016/0042-6822(90)90445-w. [DOI] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Lin J. C., Habener J. F. Structural determinants for transcriptional activation by cAMP-responsive DNA elements. J Biol Chem. 1988 Dec 5;263(34):18466–18472. [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Eaton L., Norton J. D. Independent regulation of mouse VL30 retrotransposon expression in response to serum and oncogenic cell transformation. Nucleic Acids Res. 1990 Apr 25;18(8):2069–2077. doi: 10.1093/nar/18.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavinski S., Woloschak G. E. Expression of viral and virus-like elements in DNA repair-deficient/immunodeficient "wasted" mice. J Immunol. 1989 Mar 15;142(6):1861–1866. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gromova I. I., Buchman V. L., Abagyan R. A., Ulyanov A. V., Bronstein I. B. Sequence dependent modulating effect of camptothecin on the DNA-cleaving activity of the calf thymus type I topoisomerase. Nucleic Acids Res. 1990 Feb 11;18(3):637–645. doi: 10.1093/nar/18.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K. A., Rothberg P., Kulesz-Martin M. Altered levels of endogenous retrovirus-like sequence (VL30) RNA during mouse epidermal cell carcinogenesis. Mol Carcinog. 1990;3(2):75–82. doi: 10.1002/mc.2940030205. [DOI] [PubMed] [Google Scholar]
- Hansen S. K., Nerlov C., Zabel U., Verde P., Johnsen M., Baeuerle P. A., Blasi F. A novel complex between the p65 subunit of NF-kappa B and c-Rel binds to a DNA element involved in the phorbol ester induction of the human urokinase gene. EMBO J. 1992 Jan;11(1):205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrigan M. T., Baughman G., Campbell N. F., Bourgeois S. Isolation and characterization of glucocorticoid- and cyclic AMP-induced genes in T lymphocytes. Mol Cell Biol. 1989 Aug;9(8):3438–3446. doi: 10.1128/mcb.9.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Holland G. D., King S. R., Risser R. Germ line integration of a murine leukemia provirus into a retroviruslike sequence. J Virol. 1987 Mar;61(3):701–707. doi: 10.1128/jvi.61.3.701-707.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W. S., Temin H. M. Retroviral recombination and reverse transcription. Science. 1990 Nov 30;250(4985):1227–1233. doi: 10.1126/science.1700865. [DOI] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Ransone L. J., Bengal E., Hunter T., Verma I. M. c-rel activates but v-rel suppresses transcription from kappa B sites. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam T. C., Bugge T. H., Bohm S. The long terminal repeat of VL30 retrotransposons contains sequences that determine retinoic acid-induced transcription in cultured keratinocytes. J Biol Chem. 1993 Feb 15;268(5):3251–3259. [PubMed] [Google Scholar]
- Itin A., Keshet E. Apparent recombinants between virus-liKE (VL30) and murine leukemia virus-related sequences in mouse DNA. J Virol. 1983 Jul;47(1):178–184. doi: 10.1128/jvi.47.1.178-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Schaffner W. Octamer transcription factors and the cell type-specificity of immunoglobulin gene expression. FASEB J. 1990 Mar;4(5):1444–1449. doi: 10.1096/fasebj.4.5.2407588. [DOI] [PubMed] [Google Scholar]
- Keshet E., Itin A. Patterns of genomic distribution and sequence heterogeneity of a murine "retrovirus-like" multigene family. J Virol. 1982 Jul;43(1):50–58. doi: 10.1128/jvi.43.1.50-58.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Lueders K. K. The intracisternal A-particle gene family: structure and functional aspects. Adv Cancer Res. 1988;51:183–276. doi: 10.1016/s0065-230x(08)60223-7. [DOI] [PubMed] [Google Scholar]
- Lenormand P., Pribnow D., Rodland K. D., Magun B. E. Identification of a novel enhancer element mediating calcium-dependent induction of gene expression in response to either epidermal growth factor or activation of protein kinase C. Mol Cell Biol. 1992 Jun;12(6):2793–2803. doi: 10.1128/mcb.12.6.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoSardo J. E., Boral A. L., Lenz J. Relative importance of elements within the SL3-3 virus enhancer for T-cell specificity. J Virol. 1990 Apr;64(4):1756–1763. doi: 10.1128/jvi.64.4.1756-1763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti G., Dragani T. A., Della Porta G. Cycloheximide increases endogenous retroviral RNA levels in murine liver and lung. Tumori. 1989 Jun 30;75(3):217–221. doi: 10.1177/030089168907500305. [DOI] [PubMed] [Google Scholar]
- Minty A., Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986 Jun;6(6):2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Norton J. D., Hogan B. L. Temporal and tissue-specific expression of distinct retrovirus-like (VL30) elements during mouse development. Dev Biol. 1988 Jan;125(1):226–228. doi: 10.1016/0012-1606(88)90076-0. [DOI] [PubMed] [Google Scholar]
- Orgel L. E., Crick F. H. Selfish DNA: the ultimate parasite. Nature. 1980 Apr 17;284(5757):604–607. doi: 10.1038/284604a0. [DOI] [PubMed] [Google Scholar]
- Pampeno C. L., Meruelo D. Isolation of a retroviruslike sequence from the TL locus of the C57BL/10 murine major histocompatibility complex. J Virol. 1986 May;58(2):296–306. doi: 10.1128/jvi.58.2.296-306.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panozzo J., Bertoncini D., Miller D., Libertin C. R., Woloschak G. E. Modulation of expression of virus-like elements following exposure of mice to high- and low-LET radiations. Carcinogenesis. 1991 May;12(5):801–804. doi: 10.1093/carcin/12.5.801. [DOI] [PubMed] [Google Scholar]
- Pari G., Jardine K., McBurney M. W. Multiple CArG boxes in the human cardiac actin gene promoter required for expression in embryonic cardiac muscle cells developing in vitro from embryonal carcinoma cells. Mol Cell Biol. 1991 Sep;11(9):4796–4803. doi: 10.1128/mcb.11.9.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer-Ohlsson S., Goustin A. S., Rydnert J., Wahlström T., Bjersing L., Stehelin D., Ohlsson R. Spatial and temporal pattern of cellular myc oncogene expression in developing human placenta: implications for embryonic cell proliferation. Cell. 1984 Sep;38(2):585–596. doi: 10.1016/0092-8674(84)90513-0. [DOI] [PubMed] [Google Scholar]
- Reddy M. A., Langer S. J., Colman M. S., Ostrowski M. C. An enhancer element responsive to ras and fms signaling pathways is composed of two distinct nuclear factor binding sites. Mol Endocrinol. 1992 Jul;6(7):1051–1060. doi: 10.1210/mend.6.7.1324418. [DOI] [PubMed] [Google Scholar]
- Rentrop M., Knapp B., Winter H., Schweizer J. Aminoalkylsilane-treated glass slides as support for in situ hybridization of keratin cDNAs to frozen tissue sections under varying fixation and pretreatment conditions. Histochem J. 1986 May;18(5):271–276. doi: 10.1007/BF01676237. [DOI] [PubMed] [Google Scholar]
- Rodland K. D., Muldoon L. L., Lenormand P., Magun B. E. Modulation of RNA expression by intracellular calcium. Existence of a threshold calcium concentration for induction of VL30 RNA by epidermal growth factor, endothelin, and protein kinase C. J Biol Chem. 1990 Jul 5;265(19):11000–11007. [PubMed] [Google Scholar]
- Roeser H., Doege T., Hecker E. Toxicokinetics of tumour promoters of mouse skin. II. Metabolism of the tumour promoter 12-O-tetradecanoylphorbol-13-acetate in mouse skin and biological activities of metabolites. Carcinogenesis. 1991 Sep;12(9):1563–1570. doi: 10.1093/carcin/12.9.1563. [DOI] [PubMed] [Google Scholar]
- Ross S. R., Hsu C. L., Choi Y., Mok E., Dudley J. P. Negative regulation in correct tissue-specific expression of mouse mammary tumor virus in transgenic mice. Mol Cell Biol. 1990 Nov;10(11):5822–5829. doi: 10.1128/mcb.10.11.5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. 'Solo' large terminal repeats (LTR) of an endogenous retrovirus-like gene family (VL30) in the mouse genome. Nucleic Acids Res. 1984 Mar 12;12(5):2273–2282. doi: 10.1093/nar/12.5.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman G., Itin A., Keshet E. Promoter and enhancer activities of long terminal repeats associated with cellular retrovirus-like (VL30) elements. Nucleic Acids Res. 1986 Jan 24;14(2):645–658. doi: 10.1093/nar/14.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza C., Doolittle W. F. Genes are things you have whether you want them or not. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):177–182. doi: 10.1101/sqb.1981.045.01.028. [DOI] [PubMed] [Google Scholar]
- Sartorelli V., Webster K. A., Kedes L. Muscle-specific expression of the cardiac alpha-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990 Oct;4(10):1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- Schiff R., Itin A., Keshet E. Transcriptional activation of mouse retrotransposons in vivo: specific expression in steroidogenic cells in response to trophic hormones. Genes Dev. 1991 Apr;5(4):521–532. doi: 10.1101/gad.5.4.521. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Short M. K., Okenquist S. A., Lenz J. Correlation of leukemogenic potential of murine retroviruses with transcriptional tissue preference of the viral long terminal repeats. J Virol. 1987 Apr;61(4):1067–1072. doi: 10.1128/jvi.61.4.1067-1072.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K., Saragosti S., Botchan M. Isolation of cellular genes differentially expressed in mouse NIH 3T3 cells and a simian virus 40-transformed derivative: growth-specific expression of VL30 genes. Mol Cell Biol. 1985 Oct;5(10):2590–2598. doi: 10.1128/mcb.5.10.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Golemis E., Fredrickson T. N., Hartley J. W., Hopkins N. Mutation of the core or adjacent LVb elements of the Moloney murine leukemia virus enhancer alters disease specificity. Genes Dev. 1990 Feb;4(2):233–242. doi: 10.1101/gad.4.2.233. [DOI] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Hopkins N. Point mutations in the Moloney murine leukemia virus enhancer identify a lymphoid-specific viral core motif and 1,3-phorbol myristate acetate-inducible element. J Virol. 1990 Feb;64(2):543–550. doi: 10.1128/jvi.64.2.543-550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. Sex and recombination in retroviruses. Trends Genet. 1991 Mar;7(3):71–74. doi: 10.1016/0168-9525(91)90272-R. [DOI] [PubMed] [Google Scholar]