Abstract
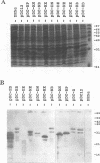
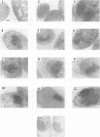
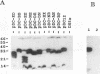
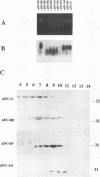
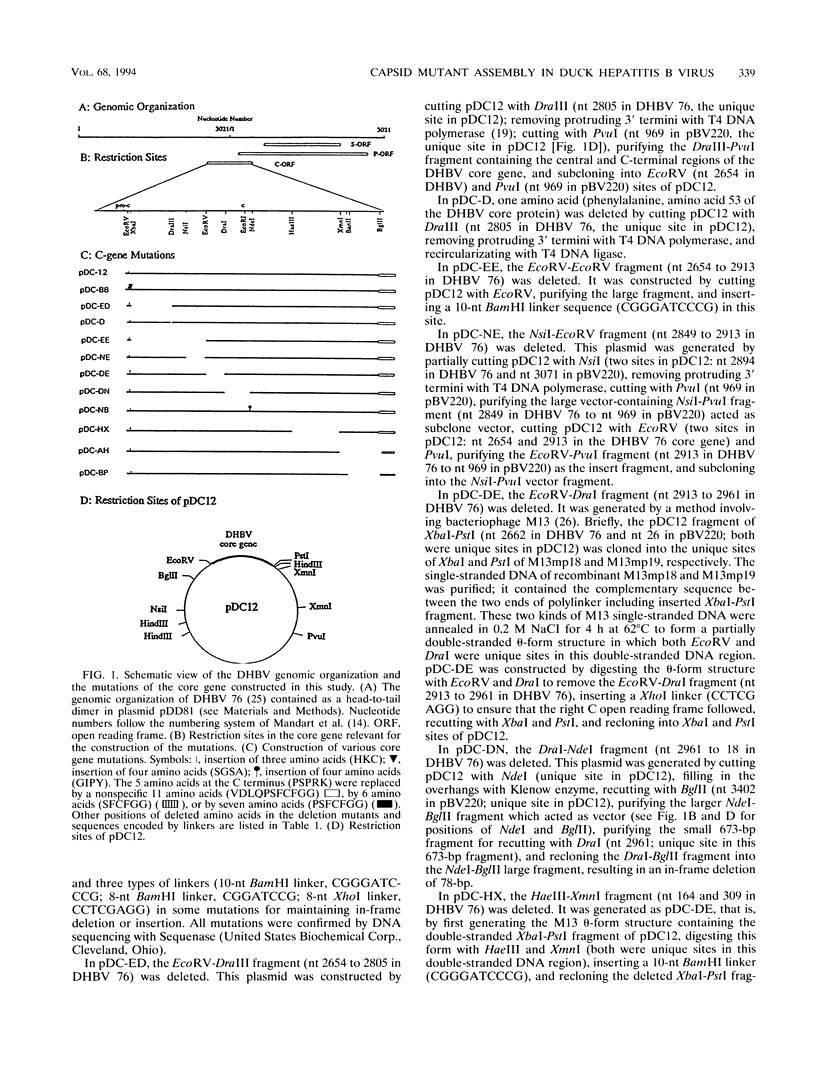
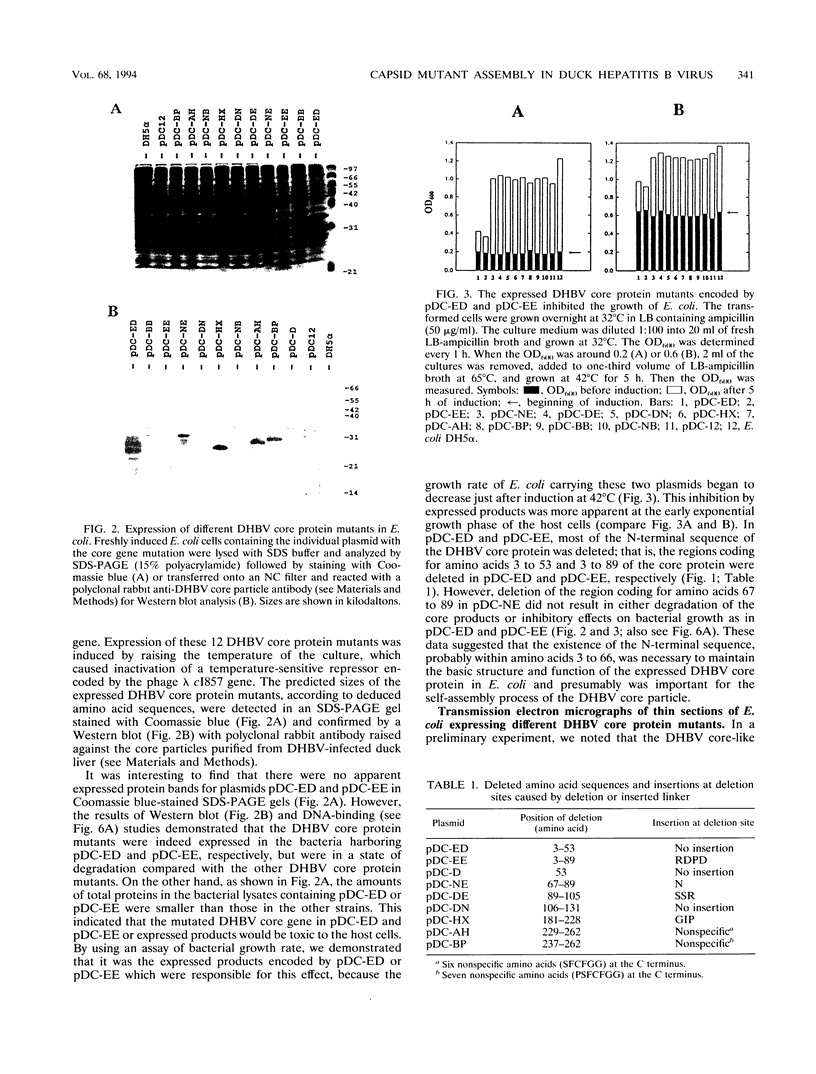
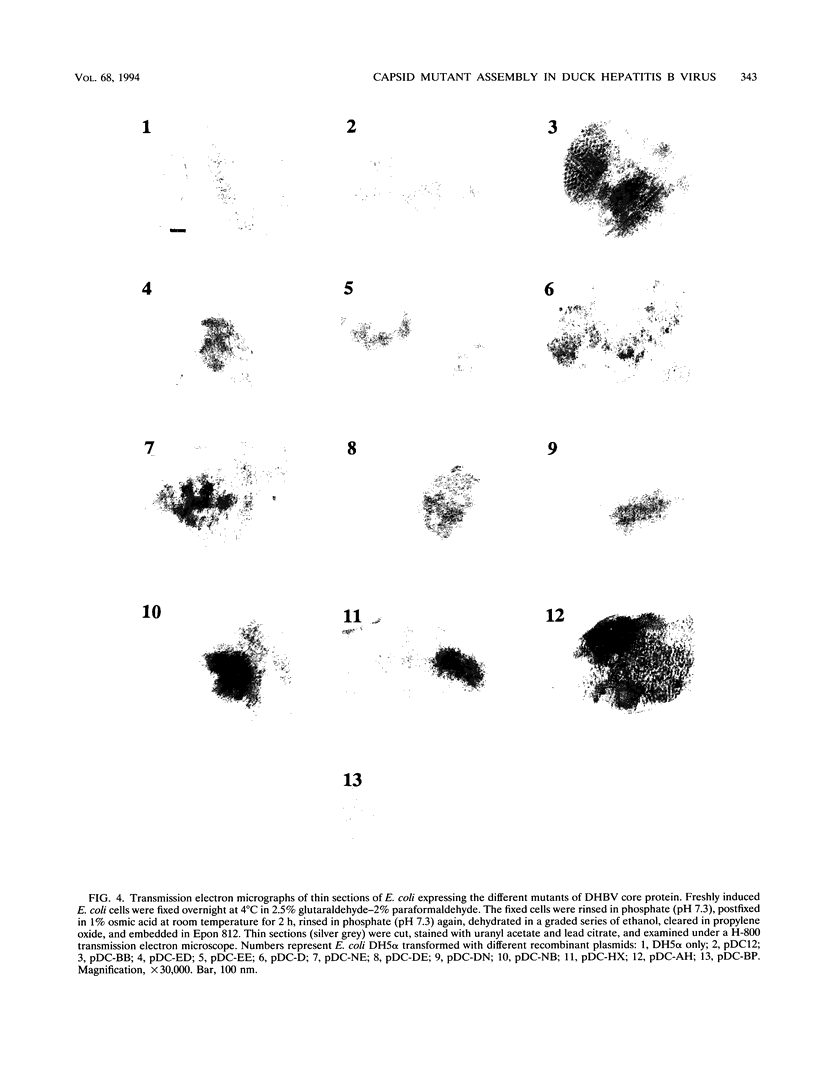
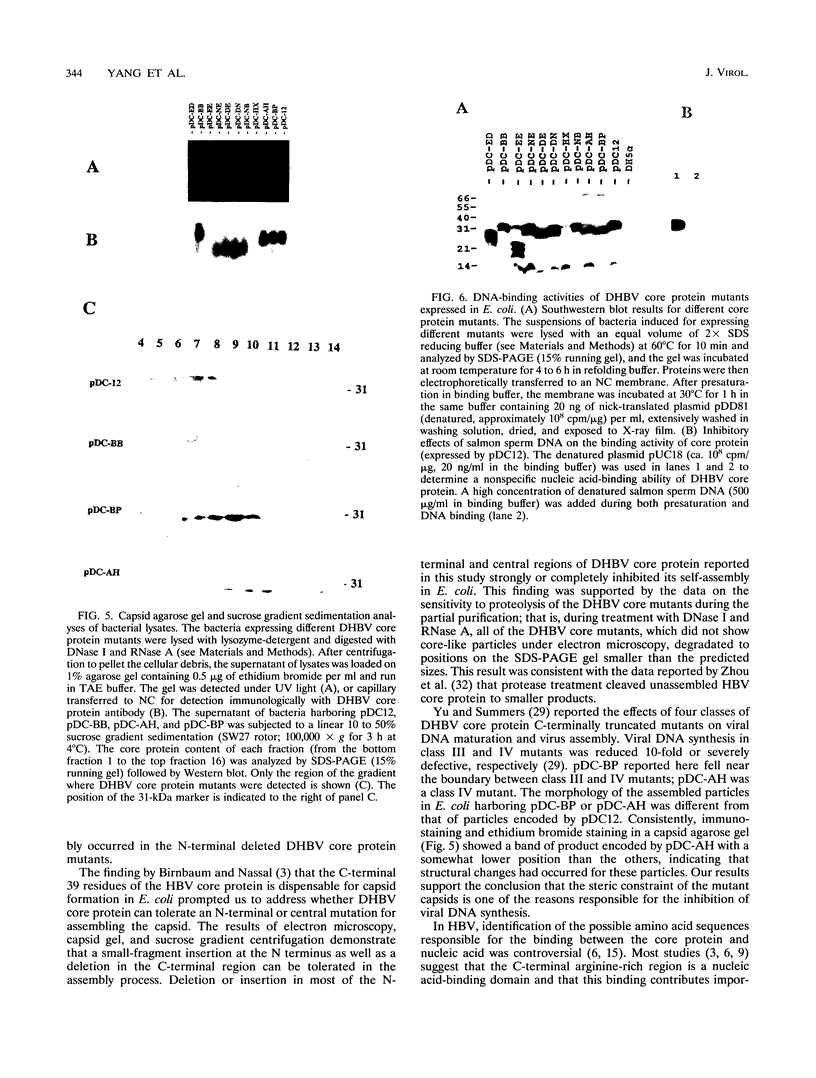
The roles of different regions of the duck hepatitis B virus (DHBV) core protein on viral capsid assembly and related functions were examined. Twelve deletion and insertion mutations which covered 80% of the DHBV C open reading frame were constructed and expressed in Escherichia coli. The N-terminal region (amino acids 3 to 66) of DHBV core protein was important for its tertiary structure and function in E. coli. The expressed core mutants without this region apparently inhibited E. coli growth. The results of transmission electron microscopy of E. coli thin sections, capsid agarose gel, and sucrose gradient sedimentation demonstrated that a few DHBV core mutants with insertion in the N terminus and deletion in the C terminus retained the ability to form core-like particles in E. coli. However, other mutations in most of N-terminal and central regions strongly inhibited the self-assembly ability of DHBV core protein in E. coli. In addition, the mutant with a C-terminal region deletion (amino acids 181 to 228) lost most of the nucleic acid-binding activity of the DHBV core protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Fuller S. D. A model for the hepatitis B virus core protein: prediction of antigenic sites and relationship to RNA virus capsid proteins. EMBO J. 1988 Mar;7(3):819–824. doi: 10.1002/j.1460-2075.1988.tb02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R., Junker-Niepmann M., Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol. 1990 Nov;64(11):5324–5332. doi: 10.1128/jvi.64.11.5324-5332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum F., Nassal M. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J Virol. 1990 Jul;64(7):3319–3330. doi: 10.1128/jvi.64.7.3319-3330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. J., Richmond J. E. Electron microscopy of hepatitis B core antigen synthesized in E. coli. Nature. 1982 Apr 15;296(5858):677–679. doi: 10.1038/296677a0. [DOI] [PubMed] [Google Scholar]
- Eckhardt S. G., Milich D. R., McLachlan A. Hepatitis B virus core antigen has two nuclear localization sequences in the arginine-rich carboxyl terminus. J Virol. 1991 Feb;65(2):575–582. doi: 10.1128/jvi.65.2.575-582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina A., Bonelli F., Zentilin L., Rindi G., Muttini M., Milanesi G. A recombinant hepatitis B core antigen polypeptide with the protamine-like domain deleted self-assembles into capsid particles but fails to bind nucleic acids. J Virol. 1989 Nov;63(11):4645–4652. doi: 10.1128/jvi.63.11.4645-4652.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Hatton T., Zhou S., Standring D. N. RNA- and DNA-binding activities in hepatitis B virus capsid protein: a model for their roles in viral replication. J Virol. 1992 Sep;66(9):5232–5241. doi: 10.1128/jvi.66.9.5232-5241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. C., Lavine J. E., Chang L. J., Varmus H. E., Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as wel as for reverse transcription. Nature. 1990 Apr 5;344(6266):552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- Hirsch R. C., Loeb D. D., Pollack J. R., Ganem D. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J Virol. 1991 Jun;65(6):3309–3316. doi: 10.1128/jvi.65.6.3309-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker-Niepmann M., Bartenschlager R., Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990 Oct;9(10):3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K., Satoh S., Ohori H. DNA-binding activity of hepatitis B e antigen polypeptide lacking the protaminelike sequence of nucleocapsid protein of human hepatitis B virus. J Virol. 1988 Sep;62(9):3517–3521. doi: 10.1128/jvi.62.9.3517-3521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassal M. Total chemical synthesis of a gene for hepatitis B virus core protein and its functional characterization. Gene. 1988 Jun 30;66(2):279–294. doi: 10.1016/0378-1119(88)90364-2. [DOI] [PubMed] [Google Scholar]
- Petit M. A., Pillot J. HBc and HBe antigenicity and DNA-binding activity of major core protein P22 in hepatitis B virus core particles isolated from the cytoplasm of human liver cells. J Virol. 1985 Feb;53(2):543–551. doi: 10.1128/jvi.53.2.543-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh J., Zweidler A., Summers J. Characterization of the major duck hepatitis B virus core particle protein. J Virol. 1989 Mar;63(3):1371–1376. doi: 10.1128/jvi.63.3.1371-1376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Bartenschlager R., Schaller H. The duck hepatitis B virus core protein contains a highly phosphorylated C terminus that is essential for replication but not for RNA packaging. J Virol. 1989 Jul;63(7):2995–3000. doi: 10.1128/jvi.63.7.2995-3000.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Schaller H. The secretory core protein of human hepatitis B virus is expressed on the cell surface. J Virol. 1989 Dec;63(12):5399–5404. doi: 10.1128/jvi.63.12.5399-5404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pourcel C., Summers J. Formation of the pool of covalently closed circular viral DNA in hepadnavirus-infected cells. Cell. 1986 Nov 7;47(3):451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- Wasenauer G., Köck J., Schlicht H. J. A cysteine and a hydrophobic sequence in the noncleaved portion of the pre-C leader peptide determine the biophysical properties of the secretory core protein (HBe protein) of human hepatitis B virus. J Virol. 1992 Sep;66(9):5338–5346. doi: 10.1128/jvi.66.9.5338-5346.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. T., Liaw Y. F., Ou J. H. The arginine-rich domain of hepatitis B virus precore and core proteins contains a signal for nuclear transport. J Virol. 1990 Dec;64(12):6141–6147. doi: 10.1128/jvi.64.12.6141-6147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C. T., Ou J. H. Phosphorylation of hepatitis B virus precore and core proteins. J Virol. 1991 May;65(5):2327–2331. doi: 10.1128/jvi.65.5.2327-2331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Summers J. A domain of the hepadnavirus capsid protein is specifically required for DNA maturation and virus assembly. J Virol. 1991 May;65(5):2511–2517. doi: 10.1128/jvi.65.5.2511-2517.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Standring D. N. Cys residues of the hepatitis B virus capsid protein are not essential for the assembly of viral core particles but can influence their stability. J Virol. 1992 Sep;66(9):5393–5398. doi: 10.1128/jvi.66.9.5393-5398.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yang S. Q., Standring D. N. Characterization of hepatitis B virus capsid particle assembly in Xenopus oocytes. J Virol. 1992 May;66(5):3086–3092. doi: 10.1128/jvi.66.5.3086-3092.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]