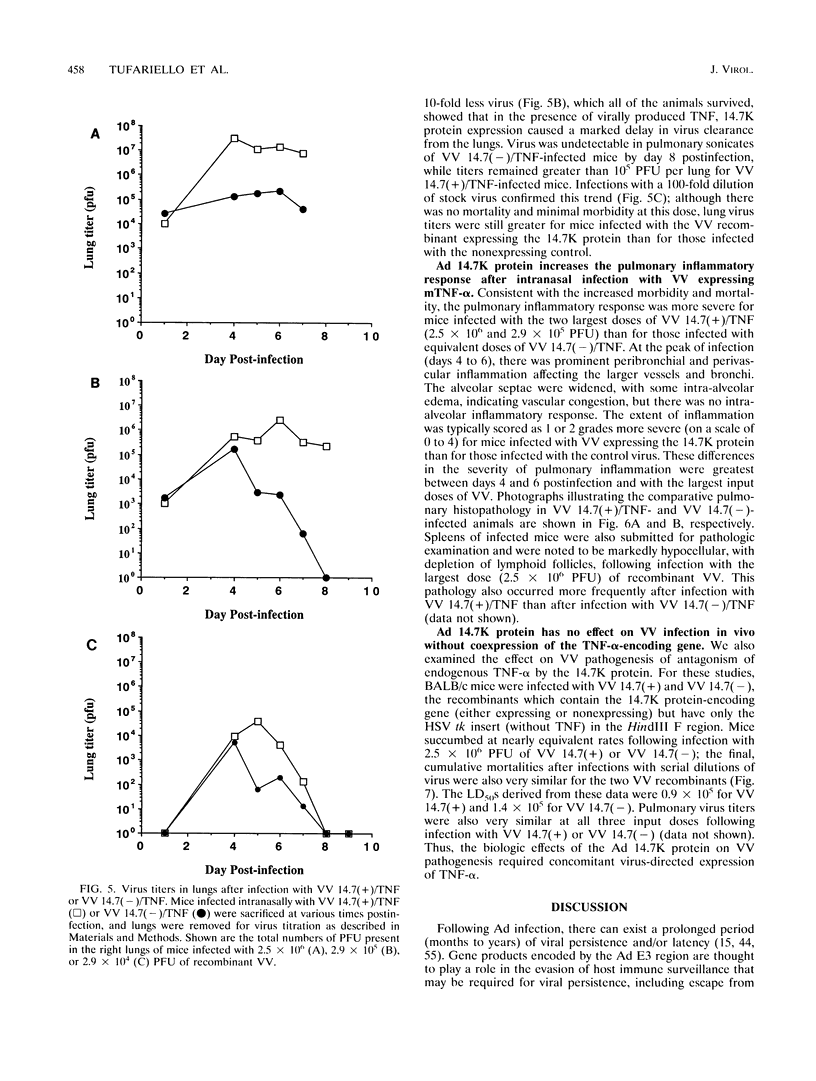
Abstract
The 14.7-kilodalton protein (14.7K protein) encoded by the adenovirus (Ad) E3 region inhibits tumor necrosis factor alpha (TNF-alpha)-mediated lysis of cells in tissue culture experiments, but the relevance of this effect in vivo is incompletely understood. To examine the effect of the ability of the Ad 14.7K protein to block TNF lysis upon viral pathogenesis in a murine model, we cloned the 14.7K protein-encoding gene into vaccinia virus (VV), permitting its study in isolation from other Ad E3 immunomodulatory proteins. The gene for murine TNF-alpha was inserted into the same VV containing the 14.7K gene to ensure that each cell infected with the VV recombinant would express both the agonist (TNF) and its antagonist (14.7K). VV was utilized as the vector because it accommodates large and multiple inserts of foreign DNA with faithful, high-level expression of the protein products. In addition, infection of mice with VV induces disease with quantifiable morbidity, mortality, and virus replication. The results of intranasal infections of BALB/c mice with these VV recombinants indicate that the Ad 14.7K protein increases the virulence of VV carrying the TNF-alpha gene by reversing the attenuating effect of TNF-alpha on VV pathogenicity. This was demonstrated by increased mortality, pulmonary pathology, and viral titers in lung tissue following infection with VV coexpressing the 14.7K protein and TNF-alpha, compared with the control virus expressing TNF-alpha alone. These results suggest that the 14.7K protein, which is nonessential for Ad replication in tissue culture, is an immunoregulatory protein which functions in vivo to help counteract the antiviral effects of TNF-alpha.
Full text
PDF

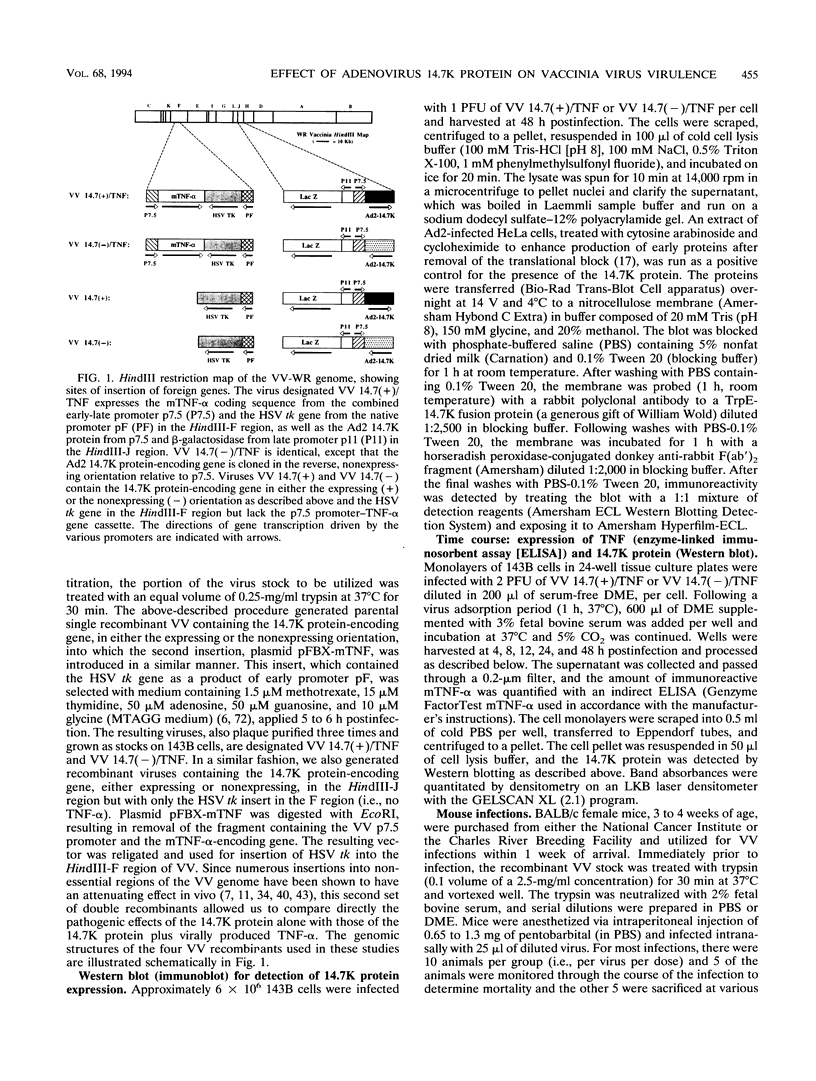
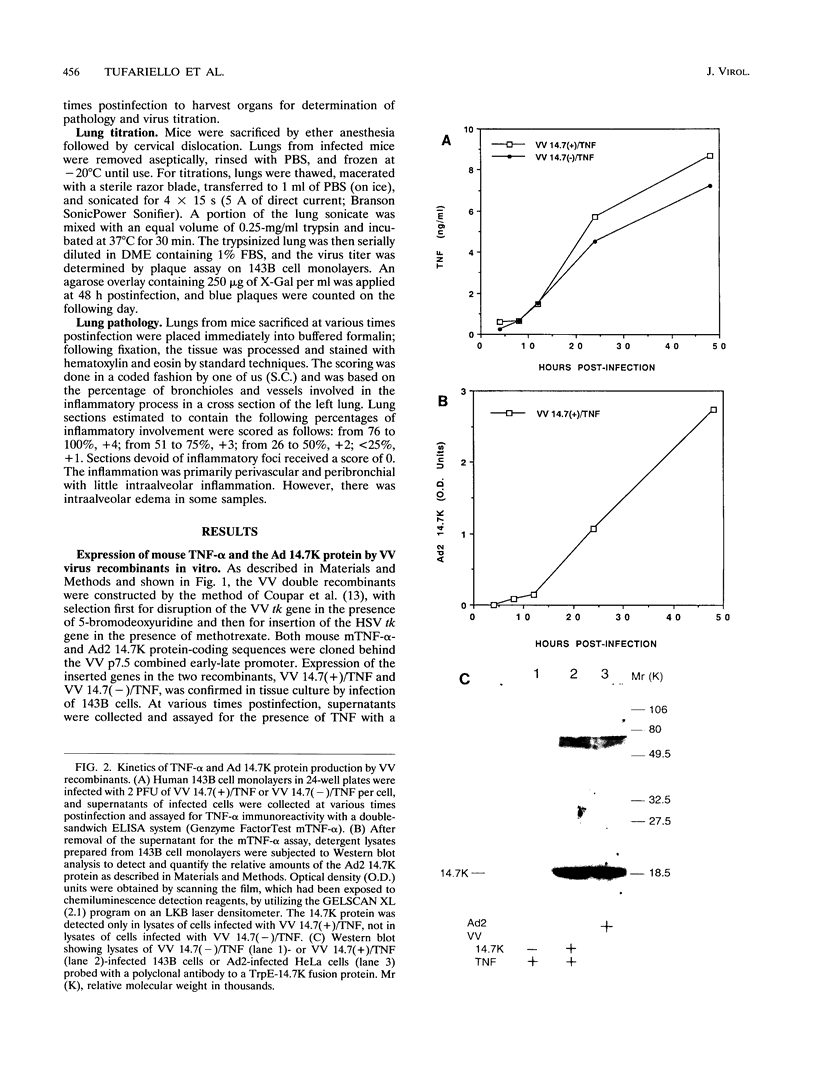
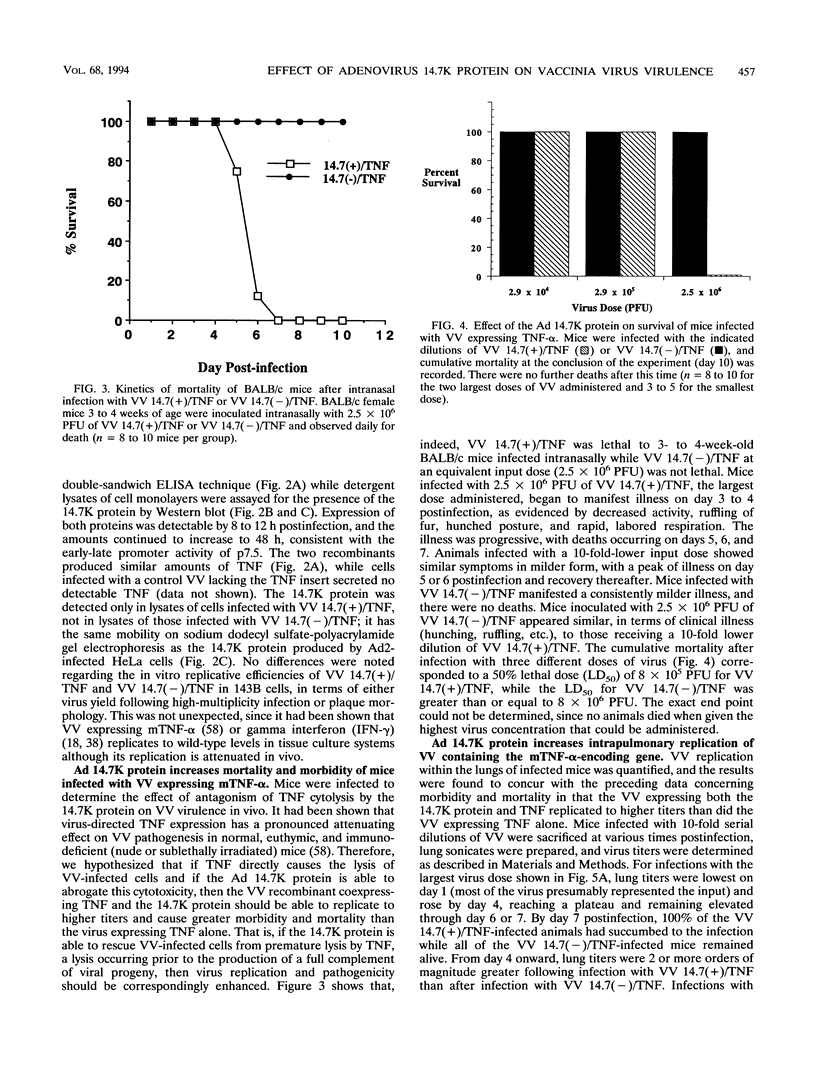
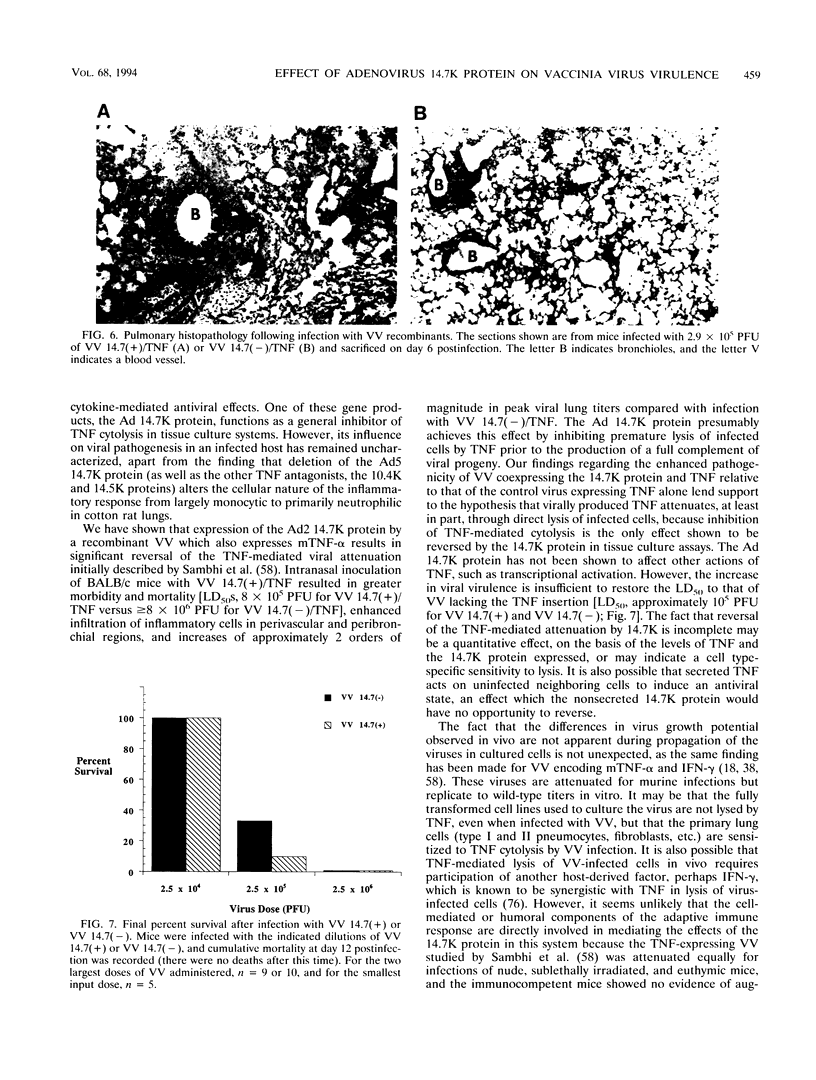



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Novick D., Hahn T., Fischer D. G., Wallach D. Increase of vulnerability to lymphotoxin in cells infected by vesicular stomatitis virus and its further augmentation by interferon. Cell Immunol. 1985 May;92(2):218–225. doi: 10.1016/0008-8749(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Aggarwal B. B., Moffat B., Harkins R. N. Human lymphotoxin. Production by a lymphoblastoid cell line, purification, and initial characterization. J Biol Chem. 1984 Jan 10;259(1):686–691. [PubMed] [Google Scholar]
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Andersson M., Päbo S., Nilsson T., Peterson P. A. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985 Nov;43(1):215–222. doi: 10.1016/0092-8674(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Arakawa T., Hsu Y. R., Toth E., Stebbing N. The antiviral activity of recombinant human tumor necrosis factor-alpha. J Interferon Res. 1987 Feb;7(1):103–105. doi: 10.1089/jir.1987.7.103. [DOI] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E. Identification and cloning of the fowlpox virus thymidine kinase gene using vaccinia virus. J Gen Virol. 1986 Aug;67(Pt 8):1591–1600. doi: 10.1099/0022-1317-67-8-1591. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Maryanski J. L., Kvist S. "E3/19K" protein of adenovirus type 2 inhibits lysis of cytolytic T lymphocytes by blocking cell-surface expression of histocompatibility class I antigens. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1356–1360. doi: 10.1073/pnas.84.5.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Child S. J., Palumbo G. J., Buller R. M., Hruby D. E. Insertional inactivation of the large subunit of ribonucleotide reductase encoded by vaccinia virus is associated with reduced virulence in vivo. Virology. 1990 Feb;174(2):625–629. doi: 10.1016/0042-6822(90)90119-c. [DOI] [PubMed] [Google Scholar]
- Cladaras C., Wold W. S. DNA sequence of the early E3 transcription unit of adenovirus 5. Virology. 1985 Jan 15;140(1):28–43. doi: 10.1016/0042-6822(85)90443-x. [DOI] [PubMed] [Google Scholar]
- Coupar B. E., Andrew M. E., Boyle D. B. A general method for the construction of recombinant vaccinia viruses expressing multiple foreign genes. Gene. 1988 Aug 15;68(1):1–10. doi: 10.1016/0378-1119(88)90593-8. [DOI] [PubMed] [Google Scholar]
- Duerksen-Hughes P., Wold W. S., Gooding L. R. Adenovirus E1A renders infected cells sensitive to cytolysis by tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4193–4200. [PubMed] [Google Scholar]
- EVANS A. S. Latent adenovirus infections of the human respiratory tract. Am J Hyg. 1958 May;67(3):256–266. doi: 10.1093/oxfordjournals.aje.a119932. [DOI] [PubMed] [Google Scholar]
- Flexner C., Hügin A., Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987 Nov 19;330(6145):259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Flomenberg P. R., Chen M., Horwitz M. S. Characterization of a major histocompatibility complex class I antigen-binding glycoprotein from adenovirus type 35, a type associated with immunocompromised hosts. J Virol. 1987 Dec;61(12):3665–3671. doi: 10.1128/jvi.61.12.3665-3671.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni L. D., Jones L., Gardner M. B., Gibson H. L., Ng C. T., Barr P. J., Yilma T. Vaccinia virus recombinants expressing chimeric proteins of human immunodeficiency virus and gamma interferon are attenuated for nude mice. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3409–3413. doi: 10.1073/pnas.89.8.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Lundholm-Beauchamp U., Horswood R. L., Pernis B., Wold W. S., Chanock R. M., Prince G. A. Role of early region 3 (E3) in pathogenesis of adenovirus disease. Proc Natl Acad Sci U S A. 1989 May;86(10):3823–3827. doi: 10.1073/pnas.86.10.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg H. S., Moldawer L. L., Sehgal P. B., Redington M., Kilian P. L., Chanock R. M., Prince G. A. A mouse model for investigating the molecular pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1651–1655. doi: 10.1073/pnas.88.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Aquino L., Duerksen-Hughes P. J., Day D., Horton T. M., Yei S. P., Wold W. S. The E1B 19,000-molecular-weight protein of group C adenoviruses prevents tumor necrosis factor cytolysis of human cells but not of mouse cells. J Virol. 1991 Jun;65(6):3083–3094. doi: 10.1128/jvi.65.6.3083-3094.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Ranheim T. S., Tollefson A. E., Aquino L., Duerksen-Hughes P., Horton T. M., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus function together to protect many but not all mouse cell lines against lysis by tumor necrosis factor. J Virol. 1991 Aug;65(8):4114–4123. doi: 10.1128/jvi.65.8.4114-4123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Sofola I. O., Tollefson A. E., Duerksen-Hughes P., Wold W. S. The adenovirus E3-14.7K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J Immunol. 1990 Nov 1;145(9):3080–3086. [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Wold W. S. Molecular mechanisms by which adenoviruses counteract antiviral immune defenses. Crit Rev Immunol. 1990;10(1):53–71. [PubMed] [Google Scholar]
- Hawkins L. K., Wold W. S. A 12,500 MW protein is coded by region E3 of adenovirus. Virology. 1992 Jun;188(2):486–494. doi: 10.1016/0042-6822(92)90502-g. [DOI] [PubMed] [Google Scholar]
- Horton T. M., Ranheim T. S., Aquino L., Kusher D. I., Saha S. K., Ware C. F., Wold W. S., Gooding L. R. Adenovirus E3 14.7K protein functions in the absence of other adenovirus proteins to protect transfected cells from tumor necrosis factor cytolysis. J Virol. 1991 May;65(5):2629–2639. doi: 10.1128/jvi.65.5.2629-2639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T. M., Tollefson A. E., Wold W. S., Gooding L. R. A protein serologically and functionally related to the group C E3 14,700-kilodalton protein is found in multiple adenovirus serotypes. J Virol. 1990 Mar;64(3):1250–1255. doi: 10.1128/jvi.64.3.1250-1255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. T., Chan Y. S., Smith G. L. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991 Feb;180(2):633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Huang S., Hendriks W., Althage A., Hemmi S., Bluethmann H., Kamijo R., Vilcek J., Zinkernagel R. M., Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993 Mar 19;259(5102):1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- Ito M., O'Malley J. A. Antiviral effects of recombinant human tumor necrosis factor. Lymphokine Res. 1987 Fall;6(4):309–318. [PubMed] [Google Scholar]
- Jättelä M. Biologic activities and mechanisms of action of tumor necrosis factor-alpha/cachectin. Lab Invest. 1991 Jun;64(6):724–742. [PubMed] [Google Scholar]
- Kerr S. M., Johnston L. H., Odell M., Duncan S. A., Law K. M., Smith G. L. Vaccinia DNA ligase complements Saccharomyces cerevisiae cdc9, localizes in cytoplasmic factories and affects virulence and virus sensitivity to DNA damaging agents. EMBO J. 1991 Dec;10(13):4343–4350. doi: 10.1002/j.1460-2075.1991.tb05012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavinskis L. S., Geckeler R., Oldstone M. B. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumour necrosis factor alpha, displays antiviral activity in vivo. J Gen Virol. 1989 Dec;70(Pt 12):3317–3325. doi: 10.1099/0022-1317-70-12-3317. [DOI] [PubMed] [Google Scholar]
- Koff W. C., Fann A. V. Human tumor necrosis factor-alpha kills herpesvirus-infected but not normal cells. Lymphokine Res. 1986 Summer;5(3):215–221. [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish M. R., King N. J., Woodhams C. E., Ramshaw I. A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990 Jan;20(1):157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Hügin A. W., Moss B. Mapping and insertional mutagenesis of a vaccinia virus gene encoding a 13,800-Da secreted protein. Virology. 1989 Aug;171(2):579–587. doi: 10.1016/0042-6822(89)90627-2. [DOI] [PubMed] [Google Scholar]
- Kriegler M., Perez C., DeFay K., Albert I., Lu S. D. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988 Apr 8;53(1):45–53. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- Kvist S., Ostberg L., Persson H., Philipson L., Peterson P. A. Molecular association between transplantation antigens and cell surface antigen in adenovirus-transformed cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5674–5678. doi: 10.1073/pnas.75.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H., Fritzsche U., Burgert H. G. Tumor necrosis factor alpha stimulates expression of adenovirus early region 3 proteins: implications for viral persistence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11857–11861. doi: 10.1073/pnas.89.24.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. S., Roos J. M., McGuigan L. C., Smith K. A., Cormier N., Cohen L. K., Roberts B. E., Payne L. G. Molecular attenuation of vaccinia virus: mutant generation and animal characterization. J Virol. 1992 May;66(5):2617–2630. doi: 10.1128/jvi.66.5.2617-2630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J. The adenovirus early proteins. Curr Top Microbiol Immunol. 1984;110:143–167. doi: 10.1007/978-3-642-46494-2_5. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Lorence R. M., Rood P. A., Kelley K. W. Newcastle disease virus as an antineoplastic agent: induction of tumor necrosis factor-alpha and augmentation of its cytotoxicity. J Natl Cancer Inst. 1988 Oct 19;80(16):1305–1312. doi: 10.1093/jnci/80.16.1305. [DOI] [PubMed] [Google Scholar]
- Mayer A., Gelderblom H., Kümel G., Jungwirth C. Interferon-gamma-induced assembly block in the replication cycle of adenovirus 2: augmentation by tumour necrosis factor-alpha. Virology. 1992 Mar;187(1):372–376. doi: 10.1016/0042-6822(92)90330-r. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HUEBNER R. J., GILMORE L. K., PARROTT R. H., WARD T. G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med. 1953 Dec;84(3):570–573. doi: 10.3181/00379727-84-20714. [DOI] [PubMed] [Google Scholar]
- Ramshaw I. A., Andrew M. E., Phillips S. M., Boyle D. B., Coupar B. E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987 Oct 8;329(6139):545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Ranheim T. S., Shisler J., Horton T. M., Wold L. J., Gooding L. R., Wold W. S. Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J Virol. 1993 Apr;67(4):2159–2167. doi: 10.1128/jvi.67.4.2159-2167.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviprakash K. S., Grunhaus A., el Kholy M. A., Horwitz M. S. The mouse adenovirus type 1 contains an unusual E3 region. J Virol. 1989 Dec;63(12):5455–5458. doi: 10.1128/jvi.63.12.5455-5458.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. R., Rodriguez D., Esteban M. Interferon treatment inhibits early events in vaccinia virus gene expression in infected mice. Virology. 1991 Dec;185(2):929–933. doi: 10.1016/0042-6822(91)90575-v. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Antonelli G., Conciatori G., Gentile M., Van Damme J., Dianzani F. The in vitro antiviral activity of tumor necrosis factor (TNF) in WISH cells is mediated by IFN-beta induction. Antiviral Res. 1989 Mar;11(2):77–88. doi: 10.1016/0166-3542(89)90010-7. [DOI] [PubMed] [Google Scholar]
- Sambhi S. K., Kohonen-Corish M. R., Ramshaw I. A. Local production of tumor necrosis factor encoded by recombinant vaccinia virus is effective in controlling viral replication in vivo. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4025–4029. doi: 10.1073/pnas.88.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Schütze S., Machleidt T., Krönke M. Mechanisms of tumor necrosis factor action. Semin Oncol. 1992 Apr;19(2 Suppl 4):16–24. [PubMed] [Google Scholar]
- Signäs C., Akusjärvi G., Pettersson U. Region E3 of human adenoviruses; differences between the oncogenic adenovirus-3 and the non-oncogenic adenovirus-2. Gene. 1986;50(1-3):173–184. doi: 10.1016/0378-1119(86)90322-7. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Soike K. F., Czarniecki C. W., Baskin G., Blanchard J., Liggitt D. Enhancement of simian varicella virus infection in African green monkeys by recombinant human tumor necrosis factor alpha. J Infect Dis. 1989 Feb;159(2):331–335. doi: 10.1093/infdis/159.2.331. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Hruby D. E., Maliszewski C. R., Pickup D. J., Sims J. E., Buller R. M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992 Oct 2;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Krajcsi P., Pursley M. H., Gooding L. R., Wold W. S. A 14,500 MW protein is coded by region E3 of group C human adenoviruses. Virology. 1990 Mar;175(1):19–29. doi: 10.1016/0042-6822(90)90182-q. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Krajcsi P., Yei S. P., Carlin C. R., Wold W. S. A 10,400-molecular-weight membrane protein is coded by region E3 of adenovirus. J Virol. 1990 Feb;64(2):794–801. doi: 10.1128/jvi.64.2.794-801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. E., Scaria A., Saha S. K., Wold W. S. The 11,600-MW protein encoded by region E3 of adenovirus is expressed early but is greatly amplified at late stages of infection. J Virol. 1992 Jun;66(6):3633–3642. doi: 10.1128/jvi.66.6.3633-3642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. E., Stewart A. R., Yei S. P., Saha S. K., Wold W. S. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus form a complex and function together to down-regulate the epidermal growth factor receptor. J Virol. 1991 Jun;65(6):3095–3105. doi: 10.1128/jvi.65.6.3095-3105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. E., Wold W. S. Identification and gene mapping of a 14,700-molecular-weight protein encoded by region E3 of group C adenoviruses. J Virol. 1988 Jan;62(1):33–39. doi: 10.1128/jvi.62.1.33-39.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992 Nov 20;258(5086):1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Lee T. H. Tumor necrosis factor. New insights into the molecular mechanisms of its multiple actions. J Biol Chem. 1991 Apr 25;266(12):7313–7316. [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Sabbatini P., Debbas M., Wold W. S., Kusher D. I., Gooding L. R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992 Jun;12(6):2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Rawls J., Saha S. K., Krajcsi P., Tollefson A. E., Gooding L. R., Wold W. S. A 6700 MW membrane protein is encoded by region E3 of adenovirus type 2. Virology. 1990 Sep;178(1):204–212. doi: 10.1016/0042-6822(90)90395-8. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Gooding L. R. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991 Sep;184(1):1–8. doi: 10.1016/0042-6822(91)90815-s. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Zilli D., Voelkel-Johnson C., Skinner T., Laster S. M. The adenovirus E3 region 14.7 kDa protein, heat and sodium arsenite inhibit the TNF-induced release of arachidonic acid. Biochem Biophys Res Commun. 1992 Oct 15;188(1):177–183. doi: 10.1016/0006-291x(92)92366-6. [DOI] [PubMed] [Google Scholar]




