Abstract
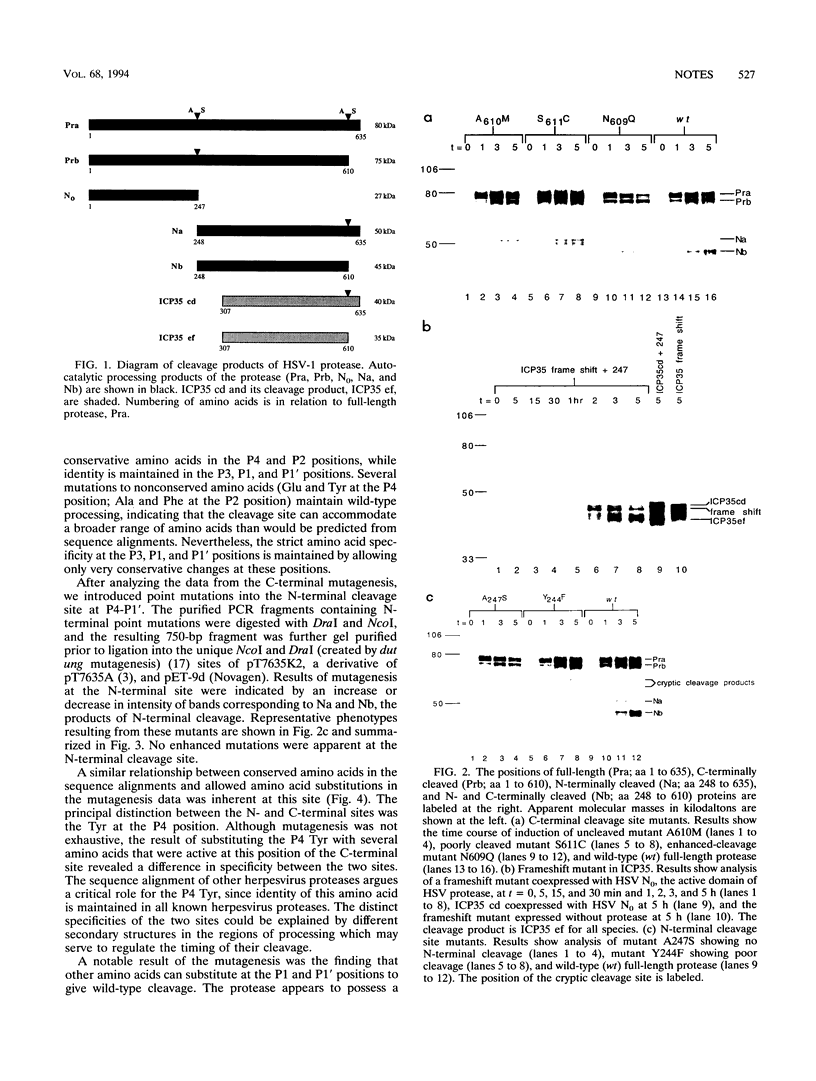
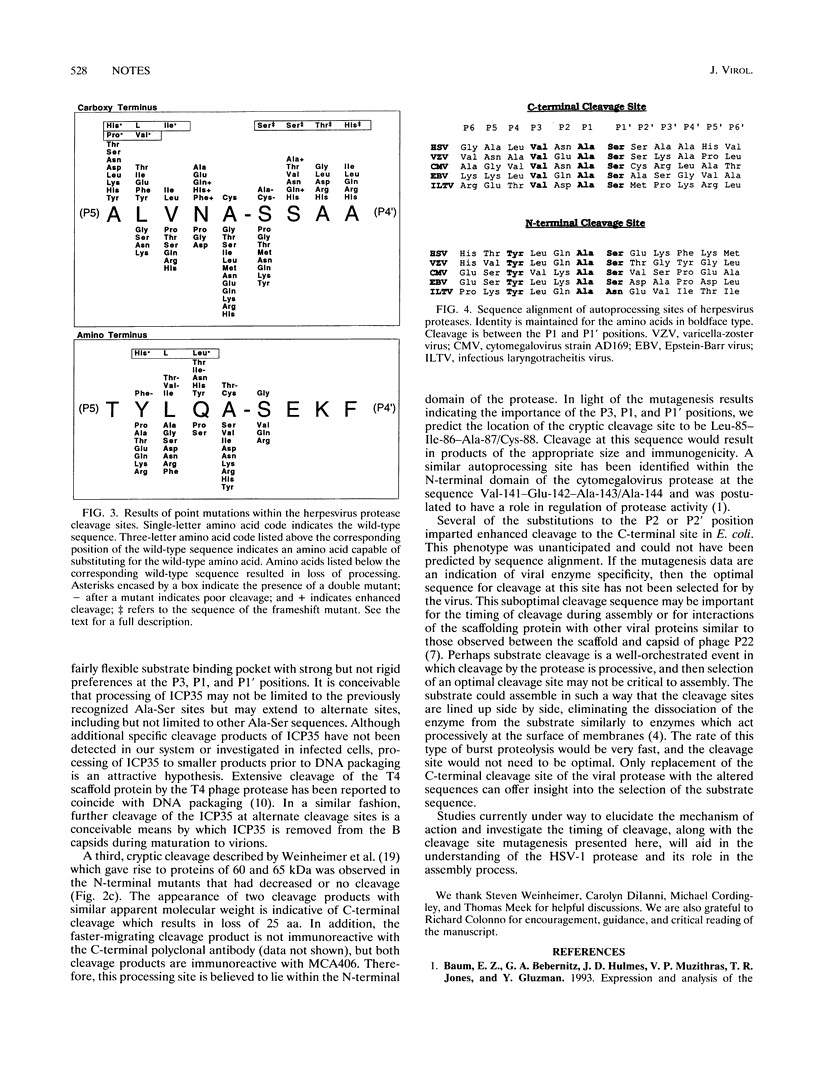
The herpes simplex virus type 1 (HSV-1) protease is cleaved at two autoprocessing sites during viral maturation, one of which shares amino acid identity with its substrate, ICP35. Similar autoprocessing sites have been observed within other members of the Herpesviridae. Introduction of point mutations within the autoprocessing sites of the HSV-1 protease indicated that specificity resides within the P4-P1' region of the cleavage sites.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deckman I. C., Hagen M., McCann P. J., 3rd Herpes simplex virus type 1 protease expressed in Escherichia coli exhibits autoprocessing and specific cleavage of the ICP35 assembly protein. J Virol. 1992 Dec;66(12):7362–7367. doi: 10.1128/jvi.66.12.7362-7367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiIanni C. L., Drier D. A., Deckman I. C., McCann P. J., 3rd, Liu F., Roizman B., Colonno R. J., Cordingley M. G. Identification of the herpes simplex virus-1 protease cleavage sites by direct sequence analysis of autoproteolytic cleavage products. J Biol Chem. 1993 Jan 25;268(3):2048–2051. [PubMed] [Google Scholar]
- Diez E., Louis-Flamberg P., Hall R. H., Mayer R. J. Substrate specificities and properties of human phospholipases A2 in a mixed vesicle model. J Biol Chem. 1992 Sep 15;267(26):18342–18348. [PubMed] [Google Scholar]
- Fuller M. T., King J. Regulation of coat protein polymerization by the scaffolding protein of bacteriophage P22. Biophys J. 1980 Oct;32(1):381–401. doi: 10.1016/S0006-3495(80)84963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Marcy A. I., Comolli J. C., Lee J. Identification of precursor to cytomegalovirus capsid assembly protein and evidence that processing results in loss of its carboxy-terminal end. J Virol. 1990 Mar;64(3):1241–1249. doi: 10.1128/jvi.64.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Casjens S. Catalytic head assembling protein in virus morphogenesis. Nature. 1974 Sep 13;251(5471):112–119. doi: 10.1038/251112a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991 Oct;65(10):5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991 Jan;65(1):206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991 Feb;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989 Nov;63(11):4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston V. G., Rixon F. J., McDougall I. M., McGregor M., al Kobaisi M. F. Processing of the herpes simplex virus assembly protein ICP35 near its carboxy terminal end requires the product of the whole of the UL26 reading frame. Virology. 1992 Jan;186(1):87–98. doi: 10.1016/0042-6822(92)90063-u. [DOI] [PubMed] [Google Scholar]
- Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Sanchez R. L., Dixon J. S., Metcalf B. W., Hassell A., Dreyer G. B., Brooks I., Debouck C. Proteolysis of an active site peptide of lactate dehydrogenase by human immunodeficiency virus type 1 protease. Biochemistry. 1992 Oct 27;31(42):10153–10168. doi: 10.1021/bi00157a003. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., McCann P. J., 3rd, O'Boyle D. R., 2nd, Stevens J. T., Boyd B. A., Drier D. A., Yamanaka G. A., DiIanni C. L., Deckman I. C., Cordingley M. G. Autoproteolysis of herpes simplex virus type 1 protease releases an active catalytic domain found in intermediate capsid particles. J Virol. 1993 Oct;67(10):5813–5822. doi: 10.1128/jvi.67.10.5813-5822.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch A. R., Woods A. S., McNally L. M., Cotter R. J., Gibson W. A herpesvirus maturational proteinase, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10792–10796. doi: 10.1073/pnas.88.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]



