Abstract
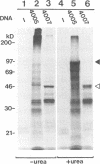
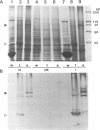
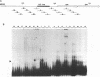
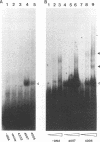
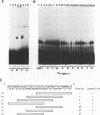
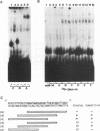
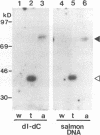
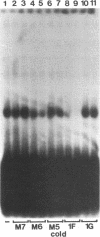
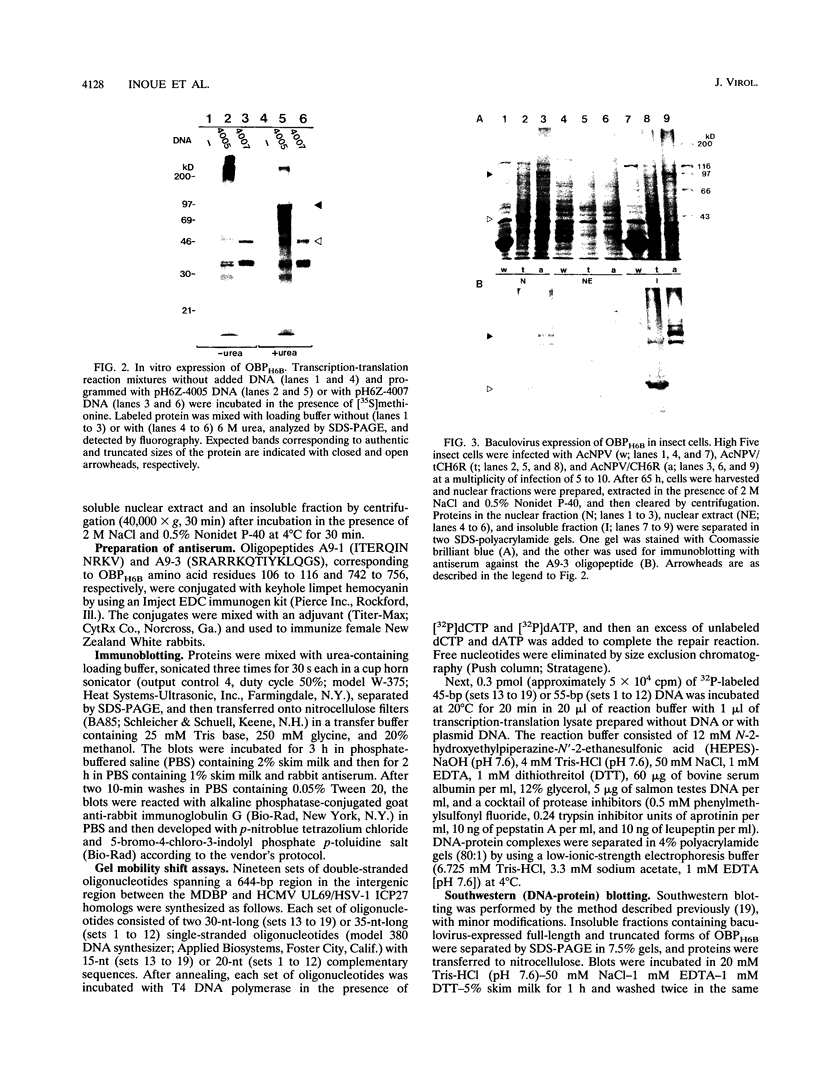
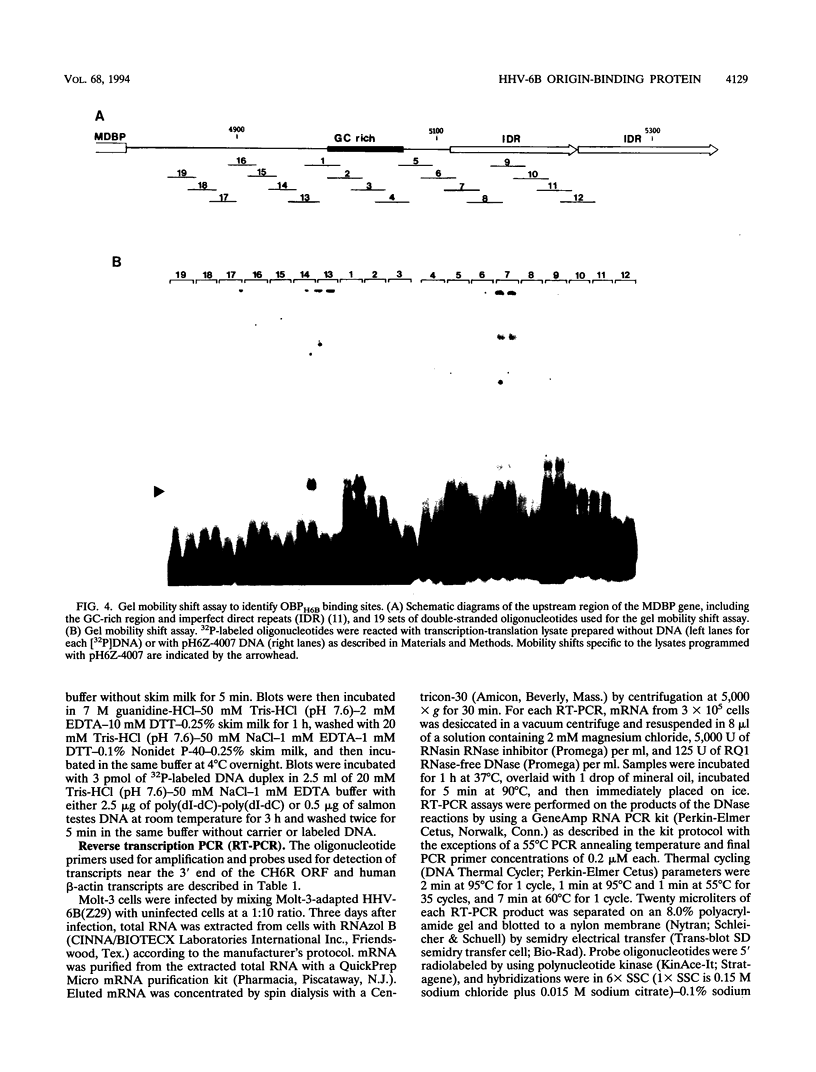
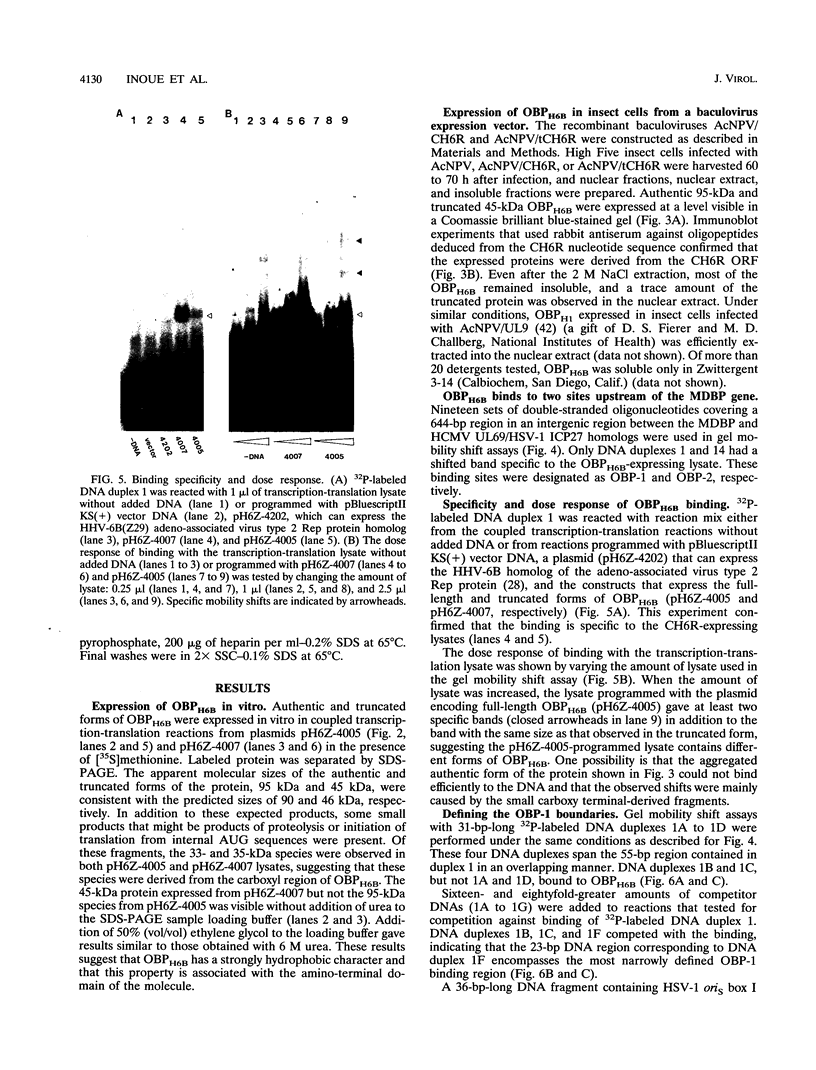
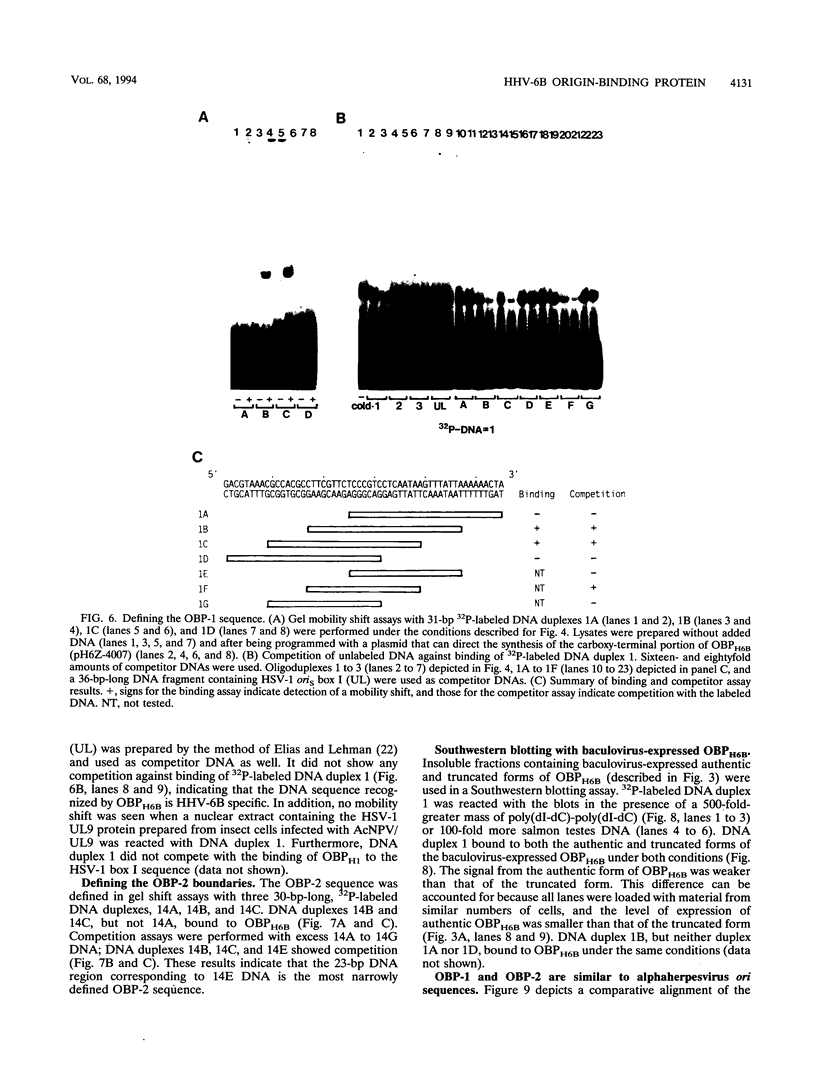
We previously identified a human herpesvirus 6B (HHV-6B) homolog of the alphaherpesvirus origin-binding protein (OBP), exemplified by the herpes simplex virus type 1 UL9 gene product. This finding is of particular interest because HHV-6B is otherwise more closely related to members of the betaherpesvirus subfamily. The prototypic betaherpesvirus, human cytomegalovirus, does not encode an obvious OBP homolog and contains a more complex origin of replication than do alphaherpesviruses. Thus, analysis of the function of the HHV-6B OBP homolog is essential for understanding the mechanism of HHV-6B DNA replication initiation. The HHV-6B OBP homolog, OBPH6B, was expressed in vitro by coupled transcription and translation and in insect cells by infection with recombinant baculoviruses. The expressed protein bound to two DNA sequences located upstream of the HHV-6B major DNA-binding protein gene homolog, within a region that was predicted to serve as an origin of replication on the basis of its sequence properties. The binding sites lie within 23-bp segments and are similar to OBP-binding sites of herpes simplex virus type 1. The two OBPH6B-binding sequences are separated by an AT-rich region and have an imperfect dyad symmetry as do the alphaherpesvirus origin regions. We identified OBPH6B transcripts by reverse transcription PCR in HHV-6B-infected Molt-3 cells. These results suggest that OBPH6B functions in a manner analogous to the alphaherpesvirus OBP and that initiation of HHV-6B DNA replication may resemble that of alphaherpesviruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Balachandran N., Josephs S. F., Hung C. L., Krueger G. R., Kramarsky B., Salahuddin S. Z., Gallo R. C. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology. 1991 Oct;184(2):545–552. doi: 10.1016/0042-6822(91)90424-a. [DOI] [PubMed] [Google Scholar]
- Anders D. G., Kacica M. A., Pari G., Punturieri S. M. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J Virol. 1992 Jun;66(6):3373–3384. doi: 10.1128/jvi.66.6.3373-3384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubin J. T., Agut H., Collandre H., Yamanishi K., Chandran B., Montagnier L., Huraux J. M. Antigenic and genetic differentiation of the two putative types of human herpes virus 6. J Virol Methods. 1993 Feb;41(2):223–234. doi: 10.1016/0166-0934(93)90129-f. [DOI] [PubMed] [Google Scholar]
- Aubin J. T., Collandre H., Candotti D., Ingrand D., Rouzioux C., Burgard M., Richard S., Huraux J. M., Agut H. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J Clin Microbiol. 1991 Feb;29(2):367–372. doi: 10.1128/jcm.29.2.367-372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann R. P., Yalamanchili V. R., O'Callaghan D. J. Functional mapping and DNA sequence of an equine herpesvirus 1 origin of replication. J Virol. 1989 Mar;63(3):1275–1283. doi: 10.1128/jvi.63.3.1275-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster A. E., Scott S. D., Sanderson M. J., Boursnell M. E., Ross N. L., Binns M. M. Gene sequence and mapping data from Marek's disease virus and herpesvirus of turkeys: implications for herpesvirus classification. J Gen Virol. 1988 Aug;69(Pt 8):2033–2042. doi: 10.1099/0022-1317-69-8-2033. [DOI] [PubMed] [Google Scholar]
- Camp H. S., Coussens P. M., Silva R. F. Cloning, sequencing, and functional analysis of a Marek's disease virus origin of DNA replication. J Virol. 1991 Nov;65(11):6320–6324. doi: 10.1128/jvi.65.11.6320-6324.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Deb S., Deb S. P. A 269-amino-acid segment with a pseudo-leucine zipper and a helix-turn-helix motif codes for the sequence-specific DNA-binding domain of herpes simplex virus type 1 origin-binding protein. J Virol. 1991 Jun;65(6):2829–2838. doi: 10.1128/jvi.65.6.2829-2838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Deb S. P. Analysis of Ori-S sequence of HSV-1: identification of one functional DNA binding domain. Nucleic Acids Res. 1989 Apr 11;17(7):2733–2752. doi: 10.1093/nar/17.7.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., Chandran B., McIntyre K., Schnabel K., Hall C. B. Phenotypic and genetic polymorphisms among human herpesvirus-6 isolates from North American infants. Virology. 1992 Sep;190(1):490–493. doi: 10.1016/0042-6822(92)91240-u. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Dollard S. C., Pellett P. E., Dambaugh T. R. Identification of a lytic-phase origin of DNA replication in human herpesvirus 6B strain Z29. J Virol. 1993 Dec;67(12):7680–7683. doi: 10.1128/jvi.67.12.7680-7683.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhurst S., McIntyre K., Schnabel K., Hall C. B. Human herpesvirus 6 (HHV-6) variant B accounts for the majority of symptomatic primary HHV-6 infections in a population of U.S. infants. J Clin Microbiol. 1993 Feb;31(2):416–418. doi: 10.1128/jcm.31.2.416-418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikstein R., Heffetz D., Ben-Neriah Y., Shaul Y. c-abl has a sequence-specific enhancer binding activity. Cell. 1992 May 29;69(5):751–757. doi: 10.1016/0092-8674(92)90287-m. [DOI] [PubMed] [Google Scholar]
- Efstathiou S., Lawrence G. L., Brown C. M., Barrell B. G. Identification of homologues to the human cytomegalovirus US22 gene family in human herpesvirus 6. J Gen Virol. 1992 Jul;73(Pt 7):1661–1671. doi: 10.1099/0022-1317-73-7-1661. [DOI] [PubMed] [Google Scholar]
- Elias P., Gustafsson C. M., Hammarsten O. The origin binding protein of herpes simplex virus 1 binds cooperatively to the viral origin of replication oris. J Biol Chem. 1990 Oct 5;265(28):17167–17173. [PubMed] [Google Scholar]
- Elias P., Lehman I. R. Interaction of origin binding protein with an origin of replication of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1988 May;85(9):2959–2963. doi: 10.1073/pnas.85.9.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., O'Donnell M. E., Mocarski E. S., Lehman I. R. A DNA binding protein specific for an origin of replication of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6322–6326. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fixman E. D., Hayward G. S., Hayward S. D. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J Virol. 1992 Aug;66(8):5030–5039. doi: 10.1128/jvi.66.8.5030-5039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U. A., Carss A. L., Sun N., Arrand J. R. Infectivity determinants encoded in a conserved gene block of human herpesvirus-6. DNA Seq. 1992;3(1):25–39. doi: 10.3109/10425179209039693. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Hazuda D. J., Perry H. C., Naylor A. M., McClements W. L. Characterization of the herpes simplex virus origin binding protein interaction with OriS. J Biol Chem. 1991 Dec 25;266(36):24621–24626. [PubMed] [Google Scholar]
- Human herpesvirus-6 strain groups: a nomenclature. Arch Virol. 1993;129(1-4):363–366. doi: 10.1007/BF01316913. [DOI] [PubMed] [Google Scholar]
- Klupp B. G., Kern H., Mettenleiter T. C. The virulence-determining genomic BamHI fragment 4 of pseudorabies virus contains genes corresponding to the UL15 (partial), UL18, UL19, UL20, and UL21 genes of herpes simplex virus and a putative origin of replication. Virology. 1992 Dec;191(2):900–908. doi: 10.1016/0042-6822(92)90265-q. [DOI] [PubMed] [Google Scholar]
- Knowles W. A., Gardner S. D. High prevalence of antibody to human herpesvirus-6 and seroconversion associated with rash in two infants. Lancet. 1988 Oct 15;2(8616):912–913. doi: 10.1016/s0140-6736(88)92522-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence G. L., Chee M., Craxton M. A., Gompels U. A., Honess R. W., Barrell B. G. Human herpesvirus 6 is closely related to human cytomegalovirus. J Virol. 1990 Jan;64(1):287–299. doi: 10.1128/jvi.64.1.287-299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquester G. J., Pellett P. E. Properties of the human herpesvirus 6 strain Z29 genome: G + C content, length, and presence of variable-length directly repeated terminal sequence elements. Virology. 1991 May;182(1):102–110. doi: 10.1016/0042-6822(91)90653-s. [DOI] [PubMed] [Google Scholar]
- Malik A. K., Martinez R., Muncy L., Carmichael E. P., Weller S. K. Genetic analysis of the herpes simplex virus type 1 UL9 gene: isolation of a LacZ insertion mutant and expression in eukaryotic cells. Virology. 1992 Oct;190(2):702–715. doi: 10.1016/0042-6822(92)90908-8. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Thomson B. J., Honess R. W., Craxton M. A., Gompels U. A., Liu M. Y., Littler E., Arrand J. R., Teo I., Jones M. D. The genome of human herpesvirus 6: maps of unit-length and concatemeric genomes for nine restriction endonucleases. J Gen Virol. 1991 Jan;72(Pt 1):157–168. doi: 10.1099/0022-1317-72-1-157. [DOI] [PubMed] [Google Scholar]
- Martinez R., Shao L., Weller S. K. The conserved helicase motifs of the herpes simplex virus type 1 origin-binding protein UL9 are important for function. J Virol. 1992 Nov;66(11):6735–6746. doi: 10.1128/jvi.66.11.6735-6746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Dolan A., McNab D., Perry L. J., Taylor P., Challberg M. D. Structures of herpes simplex virus type 1 genes required for replication of virus DNA. J Virol. 1988 Feb;62(2):444–453. doi: 10.1128/jvi.62.2.444-453.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neipel F., Ellinger K., Fleckenstein B. The unique region of the human herpesvirus 6 genome is essentially collinear with the UL segment of human cytomegalovirus. J Gen Virol. 1991 Sep;72(Pt 9):2293–2297. doi: 10.1099/0022-1317-72-9-2293. [DOI] [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus DNA replication: the UL9 gene encodes an origin-binding protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5414–5418. doi: 10.1073/pnas.85.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellett P. E., Lindquester G. J., Feorino P., Lopez C. Genomic heterogeneity of human herpesvirus 6 isolates. Adv Exp Med Biol. 1990;278:9–18. doi: 10.1007/978-1-4684-5853-4_2. [DOI] [PubMed] [Google Scholar]
- Pruksananonda P., Hall C. B., Insel R. A., McIntyre K., Pellett P. E., Long C. E., Schnabel K. C., Pincus P. H., Stamey F. R., Dambaugh T. R. Primary human herpesvirus 6 infection in young children. N Engl J Med. 1992 May 28;326(22):1445–1450. doi: 10.1056/NEJM199205283262201. [DOI] [PubMed] [Google Scholar]
- Roizmann B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Saxinger C., Polesky H., Eby N., Grufferman S., Murphy R., Tegtmeir G., Parekh V., Memon S., Hung C. Antibody reactivity with HBLV (HHV-6) in U.S. populations. J Virol Methods. 1988 Sep;21(1-4):199–208. doi: 10.1016/0166-0934(88)90066-3. [DOI] [PubMed] [Google Scholar]
- Schirmer E. C., Wyatt L. S., Yamanishi K., Rodriguez W. J., Frenkel N. Differentiation between two distinct classes of viruses now classified as human herpesvirus 6. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5922–5926. doi: 10.1073/pnas.88.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stow N. D., Davison A. J. Identification of a varicella-zoster virus origin of DNA replication and its activation by herpes simplex virus type 1 gene products. J Gen Virol. 1986 Aug;67(Pt 8):1613–1623. doi: 10.1099/0022-1317-67-8-1613. [DOI] [PubMed] [Google Scholar]
- Sánchez-Martínez D., Pellett P. E. Expression of HSV-1 and HSV-2 glycoprotein G in insect cells by using a novel baculovirus expression vector. Virology. 1991 May;182(1):229–238. doi: 10.1016/0042-6822(91)90666-y. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Thomson B. J., Efstathiou S., Honess R. W. Acquisition of the human adeno-associated virus type-2 rep gene by human herpesvirus type-6. Nature. 1991 May 2;351(6321):78–80. doi: 10.1038/351078a0. [DOI] [PubMed] [Google Scholar]
- Weir H. M., Calder J. M., Stow N. D. Binding of the herpes simplex virus type 1 UL9 gene product to an origin of viral DNA replication. Nucleic Acids Res. 1989 Feb 25;17(4):1409–1425. doi: 10.1093/nar/17.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller S. K., Spadaro A., Schaffer J. E., Murray A. W., Maxam A. M., Schaffer P. A. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol Cell Biol. 1985 May;5(5):930–942. doi: 10.1128/mcb.5.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt L. S., Balachandran N., Frenkel N. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J Infect Dis. 1990 Oct;162(4):852–857. doi: 10.1093/infdis/162.4.852. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]