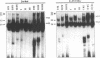Abstract
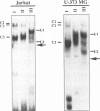
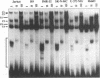
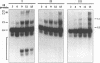
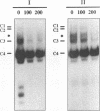
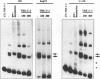
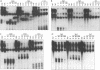
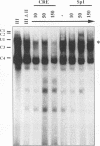
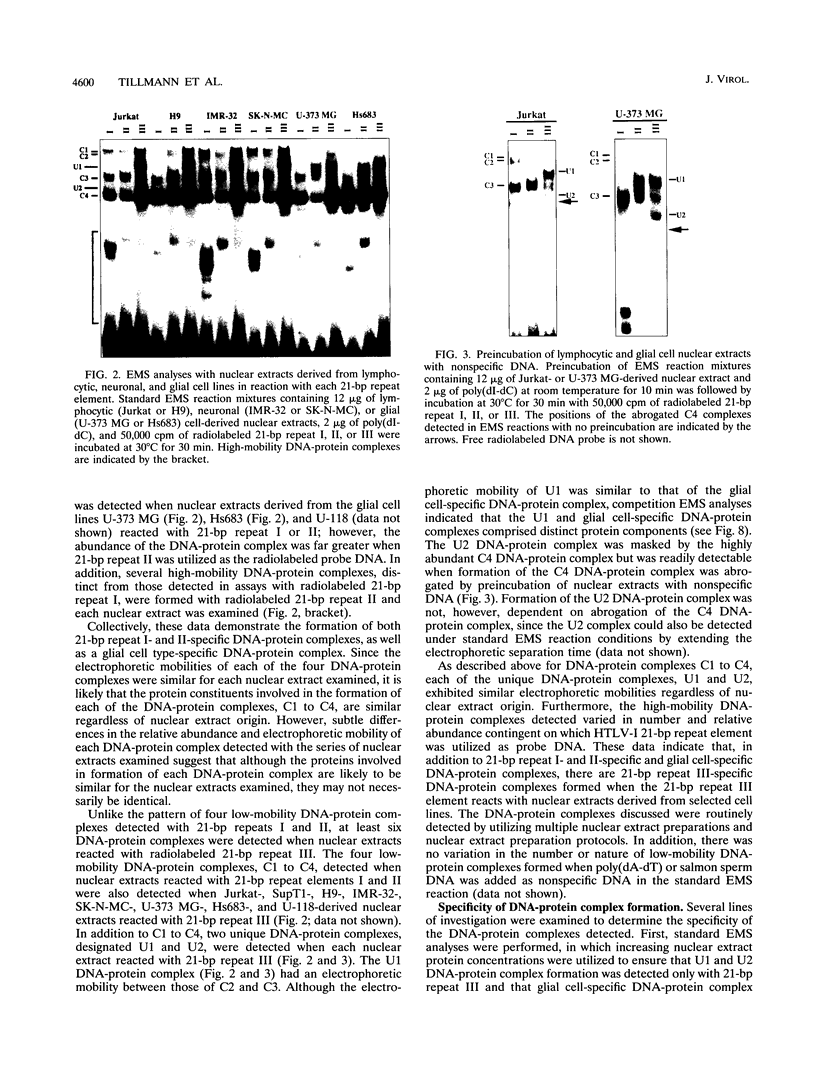
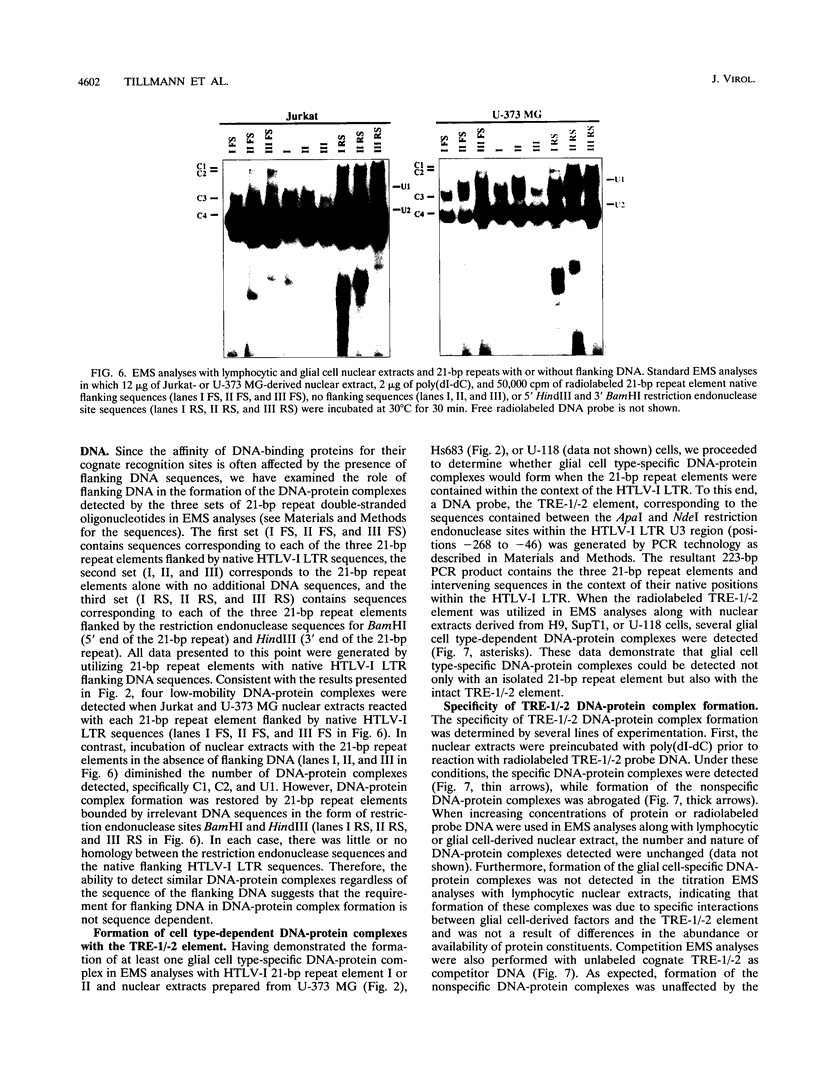
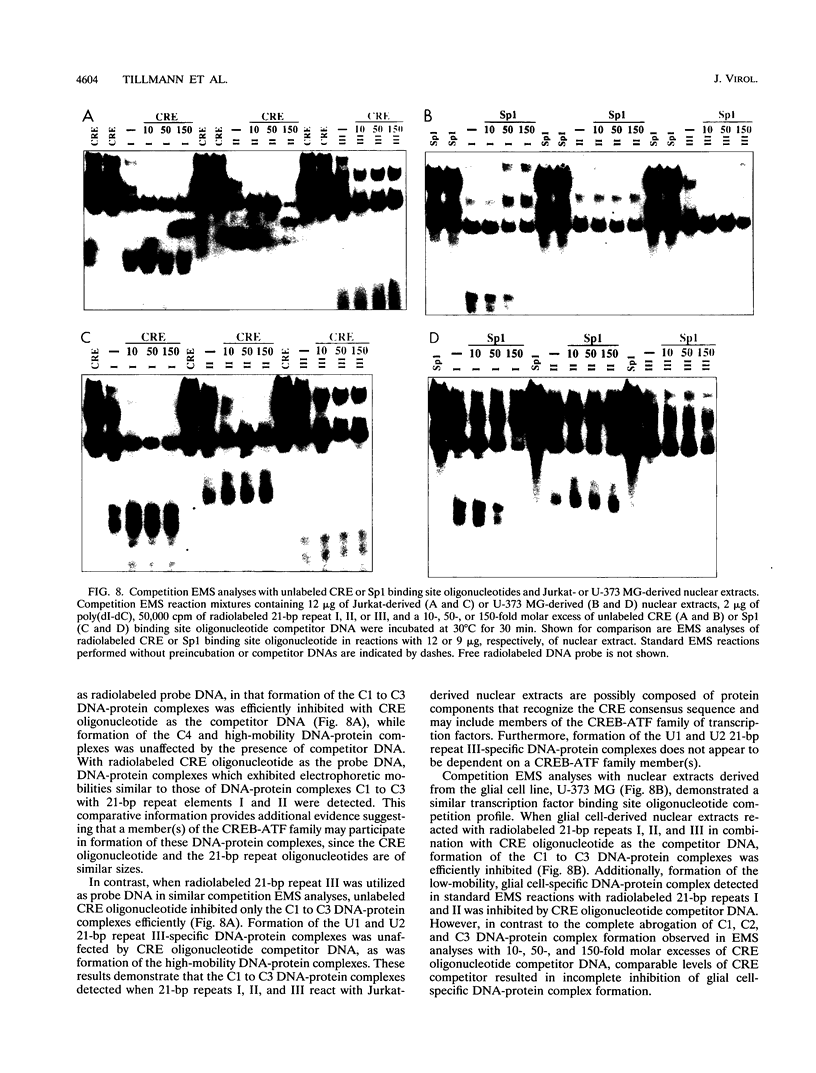
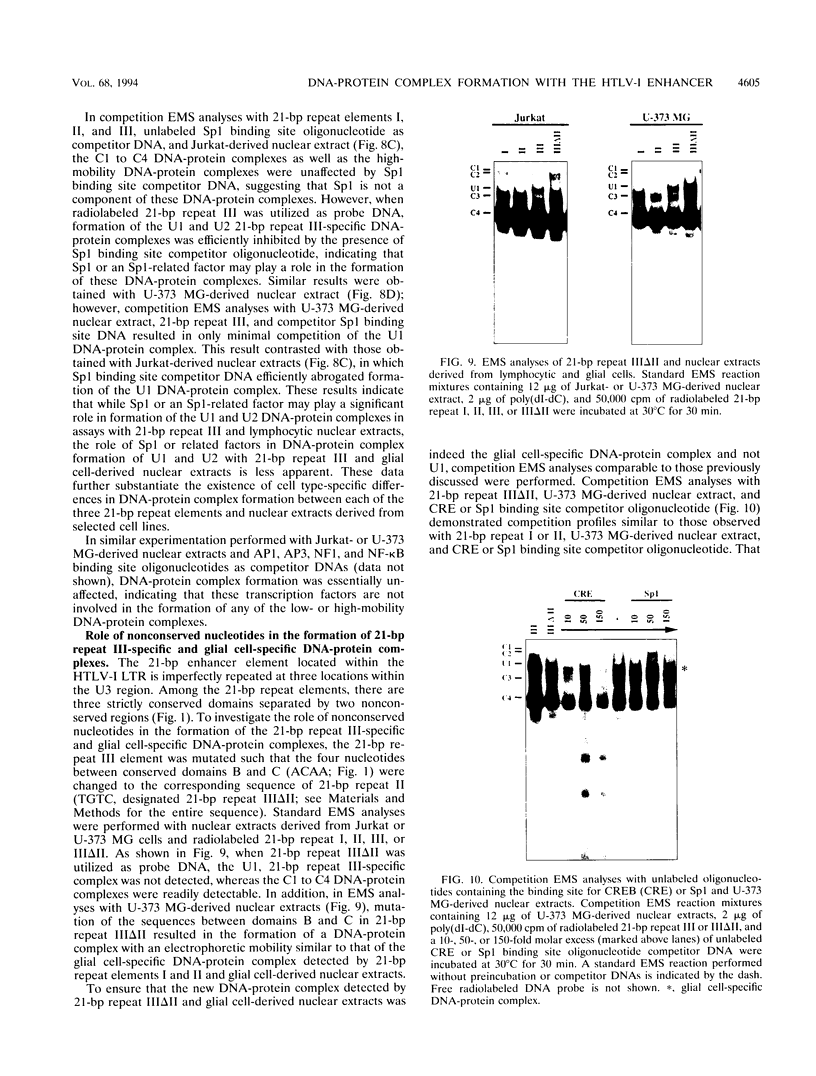
The human T-cell lymphotropic virus type I (HTLV-I)-encoded protein, Tax, is capable of trans-activating HTLV-I transcription by interacting with specific sequences in the HTLV-I long terminal repeat (LTR) which comprise an inducible enhancer containing three imperfect tandem repeats of a 21-bp sequence. There is no evidence that purified Tax can bind to DNA in the absence of cellular factors, suggesting that Tax most likely regulates transcription via interaction with cellular factors. Since HTLV-I is a documented agent of adult T-cell leukemia and tropical spastic paraparesis, disorders of the immune and nervous systems, respectively, characterization of cellular factors of lymphoid and neuroglial origin which interact with the 21-bp repeat elements is essential to understanding of the mechanisms involved in basal and Tax-mediated transcription in cells of immune and nervous system origin. Utilizing electrophoretic mobility shift (EMS) analyses, we have detected both 21-bp repeat-specific and glial cell-specific DNA-protein complexes. Several 21-bp repeat-specific DNA-protein complexes were detected when nuclear extracts derived from cells of lymphoid (Jurkat, SupT1, and H9), neuronal (IMR-32 and SK-N-MC), and glial (U-373 MG, Hs683, and U-118) origin were used in reactions with each of the three 21-bp repeat elements. In addition, a glial cell-specific DNA-protein complex was detected when nuclear extracts derived from U-373 MG, Hs683, and U-118 glial cell lines reacted with the promoter-distal and central 21-bp repeat elements. Furthermore, EMS analyses performed with nuclear extracts derived from lymphocytic and glial cell origin and a 223-bp fragment of the HTLV-I long terminal repeat encompassing the three 21-bp repeat elements (designated Tax-responsive elements 1 and 2, TRE-1/-2) have also resulted in the detection of glial cell type-specific DNA-protein complexes. Competition EMS analyses with oligonucleotides containing transcription factor binding site sequences indicate the involvement of a cyclic AMP response element binding protein in the formation of DNA-protein complexes which form with all three 21-bp repeat elements and the glial cell-specific DNA-protein complex as well as the involvement of Sp1 or an Sp1-related factor in the formation of the 21-bp repeat III-specific DNA-protein complexes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi T., Hoshida Y., Yoshino T., Teramoto N., Kondo E., Hayashi K., Takahashi K. Infectivity of human T-lymphotropic virus type I to human nervous tissue cells in vitro. Acta Neuropathol. 1992;84(2):147–152. doi: 10.1007/BF00311387. [DOI] [PubMed] [Google Scholar]
- Bessereau J. L., Mendelzon D., LePoupon C., Fiszman M., Changeux J. P., Piette J. Muscle-specific expression of the acetylcholine receptor alpha-subunit gene requires both positive and negative interactions between myogenic factors, Sp1 and GBF factors. EMBO J. 1993 Feb;12(2):443–449. doi: 10.1002/j.1460-2075.1993.tb05676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagavati S., Ehrlich G., Kula R. W., Kwok S., Sninsky J., Udani V., Poiesz B. J. Detection of human T-cell lymphoma/leukemia virus type I DNA and antigen in spinal fluid and blood of patients with chronic progressive myelopathy. N Engl J Med. 1988 May 5;318(18):1141–1147. doi: 10.1056/NEJM198805053181801. [DOI] [PubMed] [Google Scholar]
- Bhigjee A. I., Wiley C. A., Wachsman W., Amenomori T., Pirie D., Bill P. L., Windsor I. HTLV-I-associated myelopathy: clinicopathologic correlation with localization of provirus to spinal cord. Neurology. 1991 Dec;41(12):1990–1992. doi: 10.1212/wnl.41.12.1990. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brindle P. K., Montminy M. R. The CREB family of transcription activators. Curr Opin Genet Dev. 1992 Apr;2(2):199–204. doi: 10.1016/s0959-437x(05)80274-6. [DOI] [PubMed] [Google Scholar]
- Celander D., Haseltine W. A. Tissue-specific transcription preference as a determinant of cell tropism and leukaemogenic potential of murine retroviruses. Nature. 1984 Nov 8;312(5990):159–162. doi: 10.1038/312159a0. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corboy J. R., Buzy J. M., Zink M. C., Clements J. E. Expression directed from HIV long terminal repeats in the central nervous system of transgenic mice. Science. 1992 Dec 11;258(5089):1804–1808. doi: 10.1126/science.1465618. [DOI] [PubMed] [Google Scholar]
- Delmas V., Laoide B. M., Masquilier D., de Groot R. P., Foulkes N. S., Sassone-Corsi P. Alternative usage of initiation codons in mRNA encoding the cAMP-responsive-element modulator generates regulators with opposite functions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4226–4230. doi: 10.1073/pnas.89.10.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Fujisawa J., Toita M., Yoshimura T., Yoshida M. The indirect association of human T-cell leukemia virus tax protein with DNA results in transcriptional activation. J Virol. 1991 Aug;65(8):4525–4528. doi: 10.1128/jvi.65.8.4525-4528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Giam C. Z., Xu Y. L. HTLV-I tax gene product activates transcription via pre-existing cellular factors and cAMP responsive element. J Biol Chem. 1989 Sep 15;264(26):15236–15241. [PubMed] [Google Scholar]
- Gonzalez-Dunia D., Grimber G., Briand P., Brahic M., Ozden S. Tissue expression pattern directed in transgenic mice by the LTR of an HTLV-I provirus isolated from a case of tropical spastic paraparesis. Virology. 1992 Apr;187(2):705–710. doi: 10.1016/0042-6822(92)90473-3. [DOI] [PubMed] [Google Scholar]
- Hai T. W., Liu F., Coukos W. J., Green M. R. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989 Dec;3(12B):2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- Hirayama M., Miyadai T., Yokochi T., Sato K., Kubota T., Iida M., Fujiki N. Infection of human T-lymphotropic virus type I to astrocytes in vitro with induction of the class II major histocompatibility complex. Neurosci Lett. 1988 Sep 23;92(1):34–39. doi: 10.1016/0304-3940(88)90738-0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. M., Dhib-Jalbut S., Mikovits J. A., Robbins D. S., Wolf A. L., Bergey G. K., Lohrey N. C., Weislow O. S., Ruscetti F. W. Human T-cell leukemia virus type I infection of monocytes and microglial cells in primary human cultures. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11784–11788. doi: 10.1073/pnas.89.24.11784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie J. A., Matthews D. M., Cines D. B. Infection of human endothelial cells by human T-cell leukemia virus type I. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7591–7595. doi: 10.1073/pnas.81.23.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannone R., Sherman M. P., Rodgers-Johnson P. E., Beilke M. A., Mora C. A., Amin R. M., Tinsley S. R., Papsidero L. D., Poiesz B. J., Gibbs C. J., Jr HTLV-I DNA sequences in CNS tissue of a patient with tropical spastic paraparesis and HTLV-I-associated myelopathy. J Acquir Immune Defic Syndr. 1992;5(8):810–816. [PubMed] [Google Scholar]
- Kingsley C., Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol. 1992 Oct;12(10):4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kira J., Itoyama Y., Koyanagi Y., Tateishi J., Kishikawa M., Akizuki S., Kobayashi I., Toki N., Sueishi K., Sato H. Presence of HTLV-I proviral DNA in central nervous system of patients with HTLV-I-associated myelopathy. Ann Neurol. 1992 Jan;31(1):39–45. doi: 10.1002/ana.410310108. [DOI] [PubMed] [Google Scholar]
- Koralnik I. J., Lemp J. F., Jr, Gallo R. C., Franchini G. In vitro infection of human macrophages by human T-cell leukemia/lymphotropic virus type I (HTLV-I). AIDS Res Hum Retroviruses. 1992 Nov;8(11):1845–1849. doi: 10.1089/aid.1992.8.1845. [DOI] [PubMed] [Google Scholar]
- Laoide B. M., Foulkes N. S., Schlotter F., Sassone-Corsi P. The functional versatility of CREM is determined by its modular structure. EMBO J. 1993 Mar;12(3):1179–1191. doi: 10.1002/j.1460-2075.1993.tb05759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. A. Dimeric transcription factor families: it takes two to tango but who decides on partners and the venue? J Cell Sci. 1992 Sep;103(Pt 1):9–14. doi: 10.1242/jcs.103.1.9. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi B., Caronti B., Pezzella M., Bonmassar E., Lauro G. Effect of human T lymphotropic retrovirus-I exposure on cultured human glioma cell lines. Acta Neuropathol. 1991;81(6):670–674. doi: 10.1007/BF00296378. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Markowitz R. B., Dynan W. S. In vitro activation of transcription by the human T-cell leukemia virus type I Tax protein. Mol Cell Biol. 1992 May;12(5):1986–1996. doi: 10.1128/mcb.12.5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Niki M., Ohtani K., Sugamura K. Differential activation of the 21-base-pair enhancer element of human T-cell leukemia virus type I by its own trans-activator and cyclic AMP. Nucleic Acids Res. 1989 Jul 11;17(13):5207–5221. doi: 10.1093/nar/17.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg J. K., Dynan W. S. Interaction of cellular proteins with the human T-cell leukemia virus type I transcriptional control region. Purification of cellular proteins that bind the 21-base pair repeat elements. J Biol Chem. 1990 May 15;265(14):8230–8236. [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R. E., Haseltine W. A., Rosen C. A. A nuclear factor is required for transactivation of HTLV-I gene expression. Oncogene. 1988 Sep;3(3):275–279. [PubMed] [Google Scholar]
- Paskalis H., Felber B. K., Pavlakis G. N. Cis-acting sequences responsible for the transcriptional activation of human T-cell leukemia virus type I constitute a conditional enhancer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6558–6562. doi: 10.1073/pnas.83.17.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarin P. S., Robert-Gurroff M., Kalyanaraman V. S., Mann D., Minowada J., Gallo R. C. Isolation and transmission of human retrovirus (human t-cell leukemia virus). Science. 1983 Feb 18;219(4586):856–859. doi: 10.1126/science.6600519. [DOI] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Park R., Sodroski J. G., Haseltine W. A. Multiple sequence elements are required for regulation of human T-cell leukemia virus gene expression. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4919–4923. doi: 10.1073/pnas.84.14.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Haseltine W. A. Location of cis-acting regulatory sequences in the human T-cell leukemia virus type I long terminal repeat. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6502–6506. doi: 10.1073/pnas.82.19.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Inoue J., Takeda T., Yoshida M. Direct evidence that p40x of human T-cell leukemia virus type I is a trans-acting transcriptional activator. EMBO J. 1986 Mar;5(3):561–565. doi: 10.1002/j.1460-2075.1986.tb04247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takano M., Teruuchi T., Miwa M. Requirement of multiple copies of a 21-nucleotide sequence in the U3 regions of human T-cell leukemia virus type I and type II long terminal repeats for trans-acting activation of transcription. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8112–8116. doi: 10.1073/pnas.83.21.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Sodroski J. The human T-cell leukemia virus (HTLV) transactivator (Tax) protein. Biochim Biophys Acta. 1992 Sep 14;1114(1):19–29. doi: 10.1016/0304-419x(92)90003-h. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Fujisawa J. I., Toita M., Yoshida M. The trans-activator tax of human T-cell leukemia virus type 1 (HTLV-1) interacts with cAMP-responsive element (CRE) binding and CRE modulator proteins that bind to the 21-base-pair enhancer of HTLV-1. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):610–614. doi: 10.1073/pnas.90.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe K., Saida T., Kim S. U. Human and simian glial cells infected by human T-lymphotropic virus type I in culture. J Neuropathol Exp Neurol. 1989 Nov;48(6):610–619. doi: 10.1097/00005072-198911000-00003. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Seiki M., Yoshida M. HTLV type I (U. S. isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology. 1984 Feb;133(1):238–241. doi: 10.1016/0042-6822(84)90446-x. [DOI] [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. The family of human T-lymphotropic leukemia viruses: HTLV-I as the cause of adult T cell leukemia and HTLV-III as the cause of acquired immunodeficiency syndrome. Blood. 1985 Feb;65(2):253–263. [PubMed] [Google Scholar]
- Yamada M., Watabe K., Saida T., Kim S. U. Increased susceptibility of human fetal astrocytes to human T-lymphotropic virus type I in culture. J Neuropathol Exp Neurol. 1991 Mar;50(2):97–107. doi: 10.1097/00005072-199103000-00001. [DOI] [PubMed] [Google Scholar]
- Zhao L. J., Giam C. Z. Human T-cell lymphotropic virus type I (HTLV-I) transcriptional activator, Tax, enhances CREB binding to HTLV-I 21-base-pair repeats by protein-protein interaction. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7070–7074. doi: 10.1073/pnas.89.15.7070. [DOI] [PMC free article] [PubMed] [Google Scholar]