Abstract
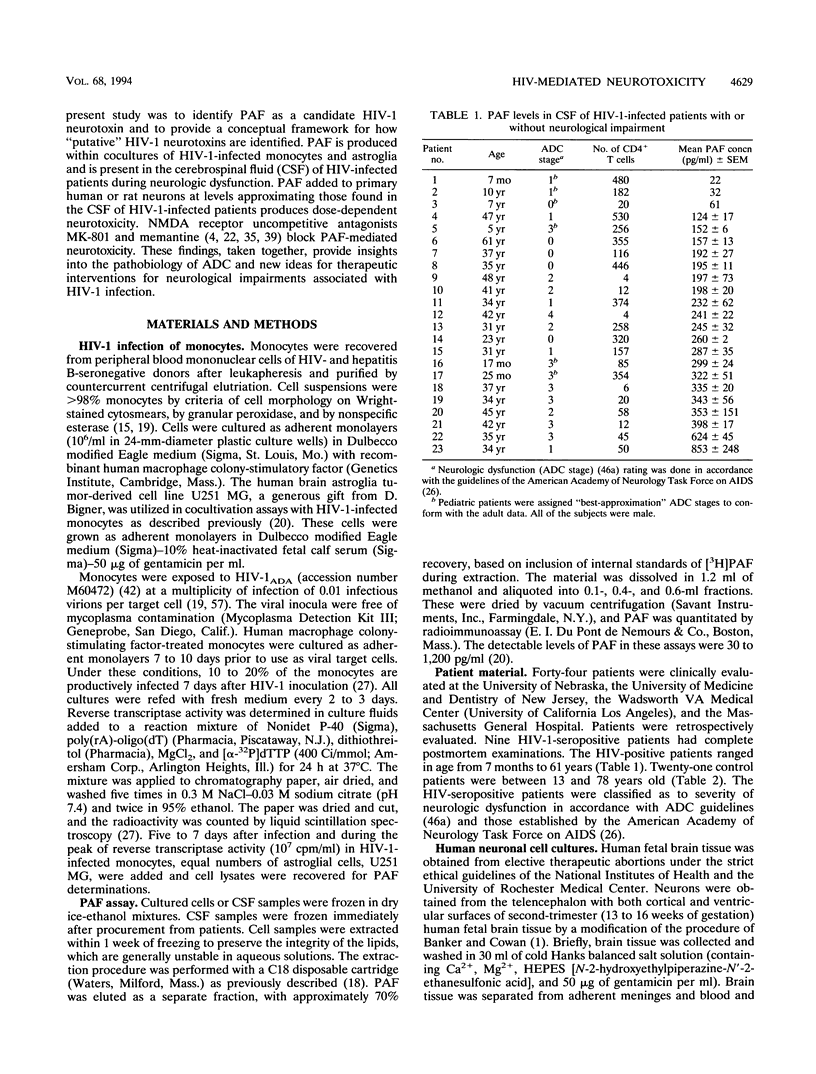
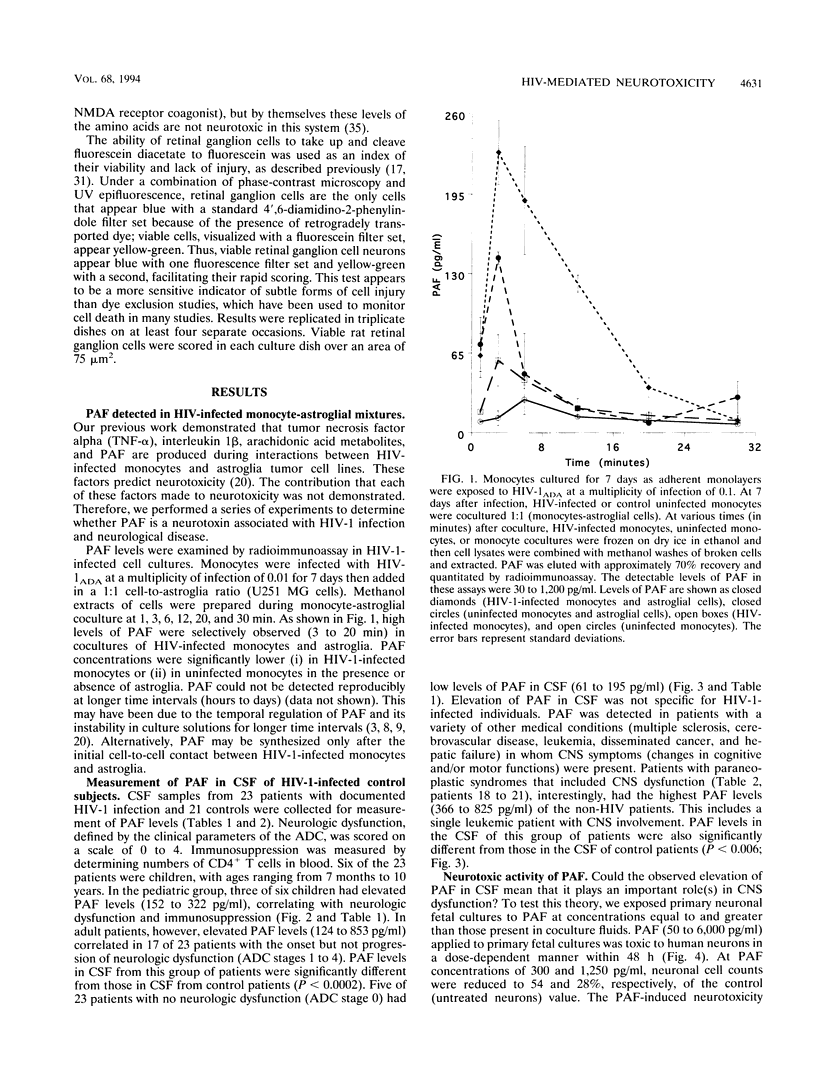
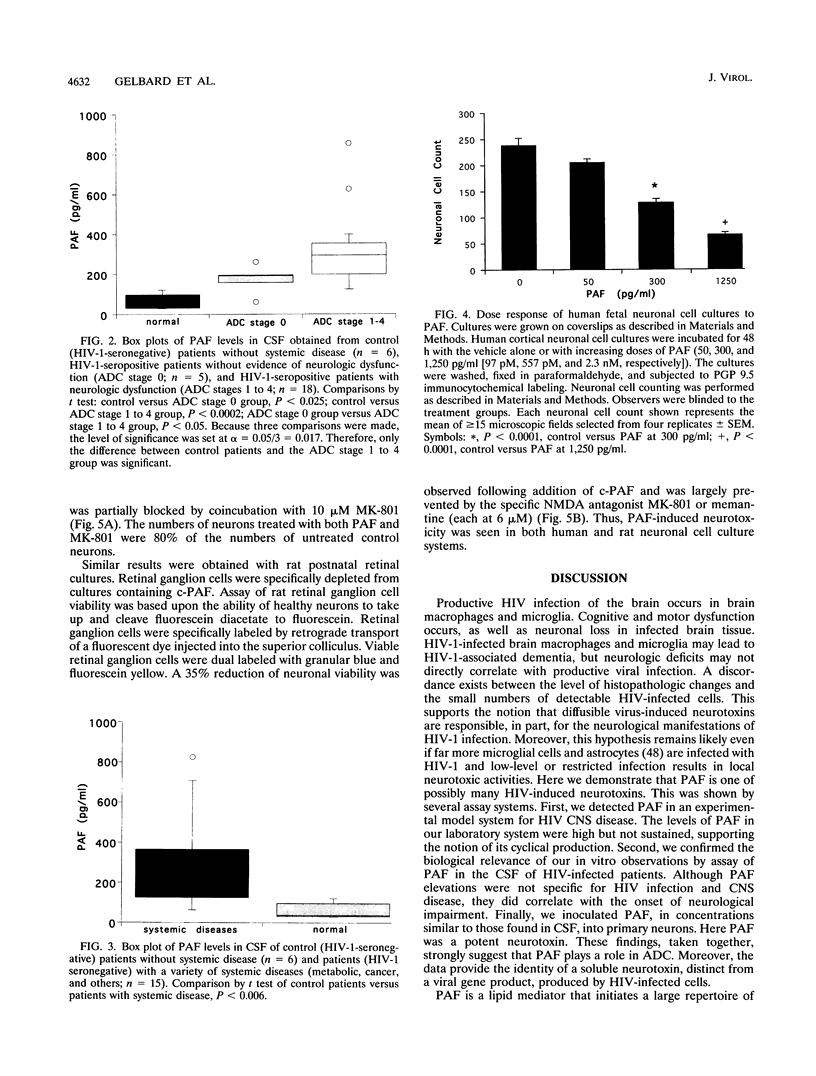
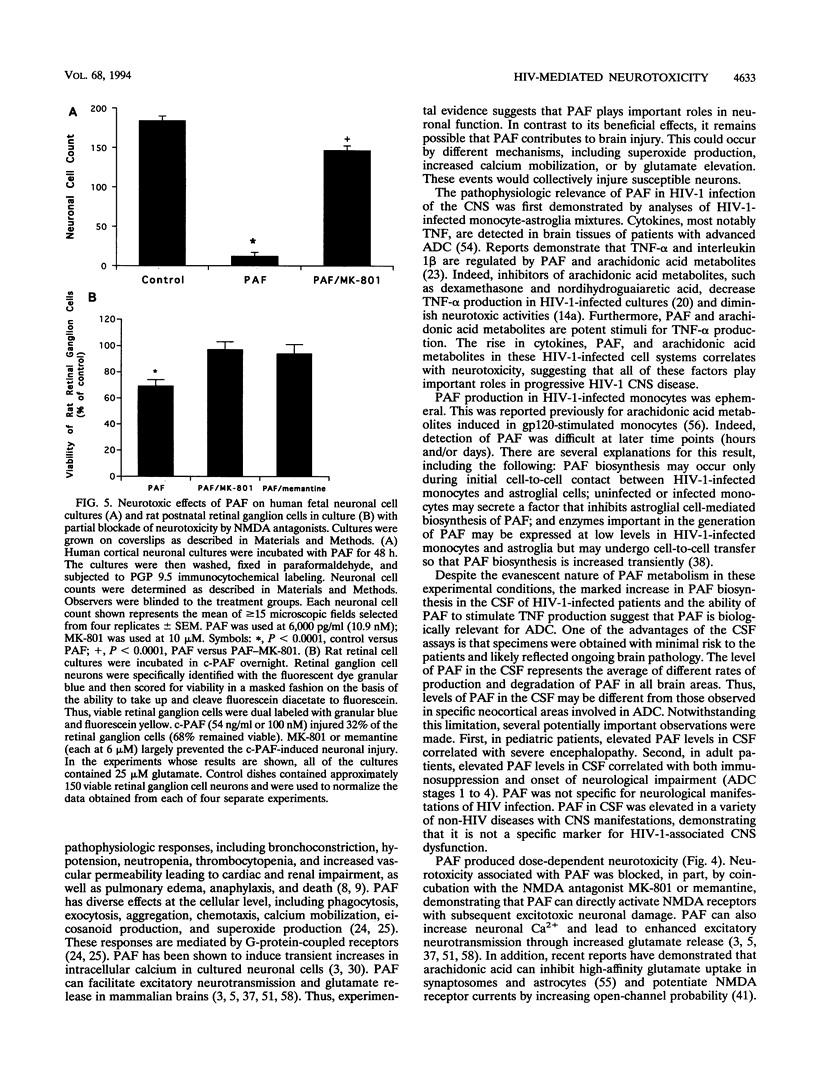
The pathogenesis of central nervous system disease during human immunodeficiency virus type 1 (HIV-1) infection revolves around productive viral infection of brain macrophages and microglia. Neuronal losses in the cortex and subcortical gray matter accompany macrophage infection. The question of how viral infection of brain macrophages ultimately leads to central nervous system (CNS) pathology remains unanswered. Our previous work demonstrated high-level production of tumor necrosis factor alpha, interleukin 1 beta, arachidonic acid metabolites, and platelet-activating factor (PAF) from HIV-infected monocytes and astroglia (H. E. Gendelman, P. Genis, M. Jett, and H. S. L. M. Nottet, in E. Major, ed., Technical Advances in AIDS Research in the Nervous System, in press; P. Genis, M. Jett, E. W. Bernton, H. A. Gelbard, K. Dzenko, R. Keane, L. Resnick, D. J. Volsky, L. G. Epstein, and H. E. Gendelman, J. Exp. Med. 176:1703-1718, 1992). These factors, together, were neurotoxic. The relative role(s) of each of these candidate neurotoxins in HIV-1-related CNS dysfunction was not unraveled by these initial experiments. We now report that PAF is produced during HIV-1-infected monocyte-astroglia interactions. PAF was detected at high levels in CSF of HIV-1-infected patients with immunosuppression and signs of CNS dysfunction. The biologic significance of the results for neurological disease was determined by addition of PAF to cultures of primary human fetal cortical or rat postnatal retinal ganglion neurons. Here, PAF at concentrations of > or = 300 pg/ml produced neuronal death. The N-methyl-D-aspartate receptor antagonist MK-801 or memantine partially blocked the neurotoxic effects of PAF. The identification of PAF as an HIV-1-induced neurotoxin provides new insights into how HIV-1 causes neurological impairment and how it may ultimately be ameliorated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banker G. A., Cowan W. M. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977 May 13;126(3):397–342. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Bryant H. U., Decoster M. A., Orenstein J. M., Ribas J. L., Meltzer M. S., Gendelman H. E. No direct neuronotoxicity by HIV-1 virions or culture fluids from HIV-1-infected T cells or monocytes. AIDS Res Hum Retroviruses. 1992 Apr;8(4):495–503. doi: 10.1089/aid.1992.8.495. [DOI] [PubMed] [Google Scholar]
- Bito H., Nakamura M., Honda Z., Izumi T., Iwatsubo T., Seyama Y., Ogura A., Kudo Y., Shimizu T. Platelet-activating factor (PAF) receptor in rat brain: PAF mobilizes intracellular Ca2+ in hippocampal neurons. Neuron. 1992 Aug;9(2):285–294. doi: 10.1016/0896-6273(92)90167-c. [DOI] [PubMed] [Google Scholar]
- Chen H. S., Pellegrini J. W., Aggarwal S. K., Lei S. Z., Warach S., Jensen F. E., Lipton S. A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992 Nov;12(11):4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark G. D., Happel L. T., Zorumski C. F., Bazan N. G. Enhancement of hippocampal excitatory synaptic transmission by platelet-activating factor. Neuron. 1992 Dec;9(6):1211–1216. doi: 10.1016/0896-6273(92)90078-r. [DOI] [PubMed] [Google Scholar]
- Cohen S. E., Mundy T., Karassik B., Lieb L., Ludwig D. D., Ward J. Neuropsychological functioning in human immunodeficiency virus type 1 seropositive children infected through neonatal blood transfusion. Pediatrics. 1991 Jul;88(1):58–68. [PubMed] [Google Scholar]
- Criscuoli M., Subissi A. Catecholamines released from the adrenal medulla exert a compensatory, protective effect at beta 2-adrenoceptors against Paf-induced death in mice. Br J Pharmacol. 1988 Jan;93(1):132–138. doi: 10.1111/j.1476-5381.1988.tb11413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darius H., Lefer D. J., Smith J. B., Lefer A. M. Role of platelet-activating factor-acether in mediating guinea pig anaphylaxis. Science. 1986 Apr 4;232(4746):58–60. doi: 10.1126/science.3082008. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Uhl G. R., Snyder S. H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer E. B., Kaiser P. K., Offermann J. T., Lipton S. A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990 Apr 20;248(4953):364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Gendelman H. E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993 May;33(5):429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Everall I. P., Luthert P. J., Lantos P. L. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991 May 11;337(8750):1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Baca L. M., Husayni H., Turpin J. A., Skillman D., Kalter D. C., Orenstein J. M., Hoover D. L., Meltzer M. S. Macrophage-HIV interaction: viral isolation and target cell tropism. AIDS. 1990 Mar;4(3):221–228. [PubMed] [Google Scholar]
- Gendelman H. E., Orenstein J. M., Martin M. A., Ferrua C., Mitra R., Phipps T., Wahl L. A., Lane H. C., Fauci A. S., Burke D. S. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988 Apr 1;167(4):1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Vaca K., Noonan C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990 Dec 14;250(4987):1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Hahn J. S., Aizenman E., Lipton S. A. Central mammalian neurons normally resistant to glutamate toxicity are made sensitive by elevated extracellular Ca2+: toxicity is blocked by the N-methyl-D-aspartate antagonist MK-801. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6556–6560. doi: 10.1073/pnas.85.17.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Production of tumor necrosis factor by rIFN-gamma-primed C3H/HeJ (Lpsd) macrophages requires the presence of lipid A-associated proteins. J Immunol. 1988 Dec 15;141(12):4196–4202. [PubMed] [Google Scholar]
- Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991 Jan 24;349(6307):342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- Hwang S. B. Specific receptors of platelet-activating factor, receptor heterogeneity, and signal transduction mechanisms. J Lipid Mediat. 1990 May-Jul;2(3-4):123–158. [PubMed] [Google Scholar]
- Kalter D. C., Nakamura M., Turpin J. A., Baca L. M., Hoover D. L., Dieffenbach C., Ralph P., Gendelman H. E., Meltzer M. S. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991 Jan 1;146(1):298–306. [PubMed] [Google Scholar]
- Kent C., Clarke P. J. The immunolocalisation of the neuroendocrine specific protein PGP9.5 during neurogenesis in the rat. Brain Res Dev Brain Res. 1991 Jan 15;58(1):147–150. doi: 10.1016/0165-3806(91)90248-h. [DOI] [PubMed] [Google Scholar]
- Ketzler S., Weis S., Haug H., Budka H. Loss of neurons in the frontal cortex in AIDS brains. Acta Neuropathol. 1990;80(1):92–94. doi: 10.1007/BF00294228. [DOI] [PubMed] [Google Scholar]
- Leifer D., Lipton S. A., Barnstable C. J., Masland R. H. Monoclonal antibody to Thy-1 enhances regeneration of processes by rat retinal ganglion cells in culture. Science. 1984 Apr 20;224(4646):303–306. doi: 10.1126/science.6143400. [DOI] [PubMed] [Google Scholar]
- Lindgren J. A., Hökfelt T., Dahlén S. E., Patrono C., Samuelsson B. Leukotrienes in the rat central nervous system. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6212–6216. doi: 10.1073/pnas.81.19.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A. Human immunodeficiency virus-infected macrophages, gp120, and N-methyl-D-aspartate receptor-mediated neurotoxicity. Ann Neurol. 1993 Feb;33(2):227–228. doi: 10.1002/ana.410330218. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Sucher N. J., Kaiser P. K., Dreyer E. B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991 Jul;7(1):111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Tauck D. L. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987 Apr;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Islam N., Broekman M. J., Falck J. R., Fischer S., von Schacky C. Platelet-neutrophil interactions. (12S)-hydroxyeicosatetraen-1,20-dioic acid: a new eicosanoid synthesized by unstimulated neutrophils from (12S)-20-dihydroxyeicosatetraenoic acid. J Biol Chem. 1988 Feb 15;263(5):2223–2229. [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Michaels J., Sharer L. R., Epstein L. G. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1(1):71–104. [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Navia B. A., Jordan B. D., Price R. W. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986 Jun;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology. 1991 Jun;41(6):778–785. doi: 10.1212/wnl.41.6.778. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Redman J. F., Jr, Schmitt J. D., Ellis J. M., Surles J. R., Marx M. H., Piantadosi C., Wykle R. L. 1-O-alkyl-2-N-methylcarbamyl-glycerophosphocholine: a biologically potent, non-metabolizable analog of platelet-activating factor. Biochem Biophys Res Commun. 1987 Aug 31;147(1):18–24. doi: 10.1016/s0006-291x(87)80081-5. [DOI] [PubMed] [Google Scholar]
- Poubelle P. E., Gingras D., Demers C., Dubois C., Harbour D., Grassi J., Rola-Pleszczynski M. Platelet-activating factor (PAF-acether) enhances the concomitant production of tumour necrosis factor-alpha and interleukin-1 by subsets of human monocytes. Immunology. 1991 Feb;72(2):181–187. [PMC free article] [PubMed] [Google Scholar]
- Price R. W., Brew B. J. The AIDS dementia complex. J Infect Dis. 1988 Nov;158(5):1079–1083. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- Pulliam L., Herndier B. G., Tang N. M., McGrath M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J Clin Invest. 1991 Feb;87(2):503–512. doi: 10.1172/JCI115024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Sharer L. R., Epstein L. G., Michaels J., Mintz M., Louder M., Golding K., Cvetkovich T. A., Blumberg B. M. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994 Mar;44(3 Pt 1):474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Epstein L. G., Cho E. S., Joshi V. V., Meyenhofer M. F., Rankin L. F., Petito C. K. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986 Mar;17(3):271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Sharer L. R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992 Jan;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Shukla S. D. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992 Mar;6(6):2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- Tardieu M., Héry C., Peudenier S., Boespflug O., Montagnier L. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann Neurol. 1992 Jul;32(1):11–17. doi: 10.1002/ana.410320104. [DOI] [PubMed] [Google Scholar]
- Tenhula W. N., Xu S. Z., Madigan M. C., Heller K., Freeman W. R., Sadun A. A. Morphometric comparisons of optic nerve axon loss in acquired immunodeficiency syndrome. Am J Ophthalmol. 1992 Jan 15;113(1):14–20. doi: 10.1016/s0002-9394(14)75746-0. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Volterra A., Trotti D., Cassutti P., Tromba C., Salvaggio A., Melcangi R. C., Racagni G. High sensitivity of glutamate uptake to extracellular free arachidonic acid levels in rat cortical synaptosomes and astrocytes. J Neurochem. 1992 Aug;59(2):600–606. doi: 10.1111/j.1471-4159.1992.tb09411.x. [DOI] [PubMed] [Google Scholar]
- Wahl L. M., Corcoran M. L., Pyle S. W., Arthur L. O., Harel-Bellan A., Farrar W. L. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc Natl Acad Sci U S A. 1989 Jan;86(2):621–625. doi: 10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieraszko A., Li G., Kornecki E., Hogan M. V., Ehrlich Y. H. Long-term potentiation in the hippocampus induced by platelet-activating factor. Neuron. 1993 Mar;10(3):553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- Wieraszko A., Li G., Kornecki E., Hogan M. V., Ehrlich Y. H. Long-term potentiation in the hippocampus induced by platelet-activating factor. Neuron. 1993 Mar;10(3):553–557. doi: 10.1016/0896-6273(93)90342-o. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Masliah E., Morey M., Lemere C., DeTeresa R., Grafe M., Hansen L., Terry R. Neocortical damage during HIV infection. Ann Neurol. 1991 Jun;29(6):651–657. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]


