Abstract
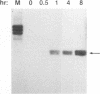
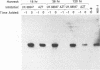
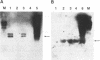
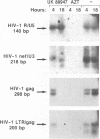
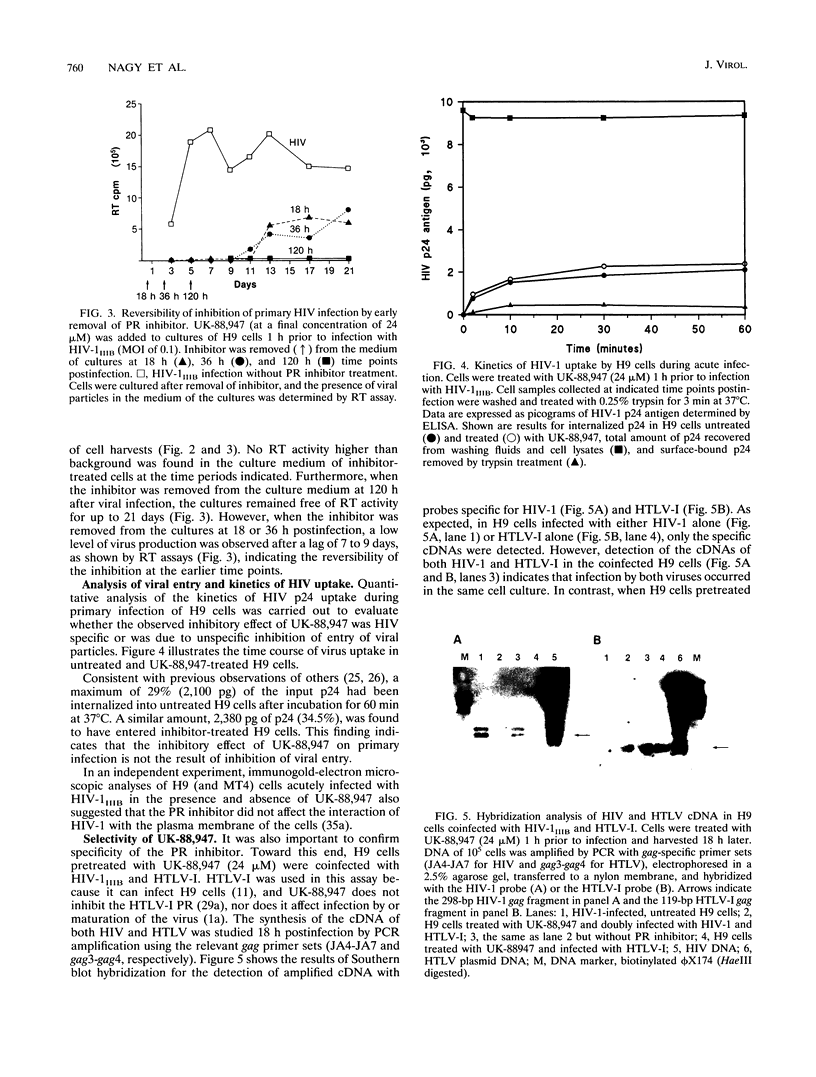
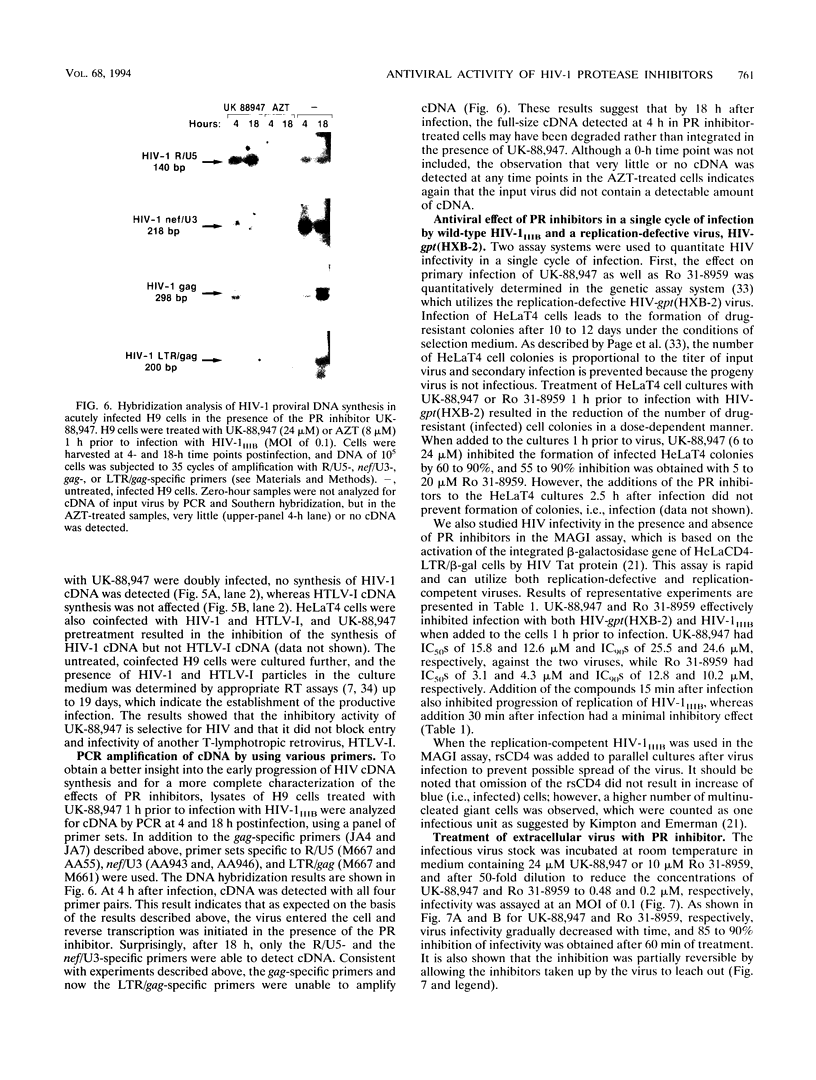
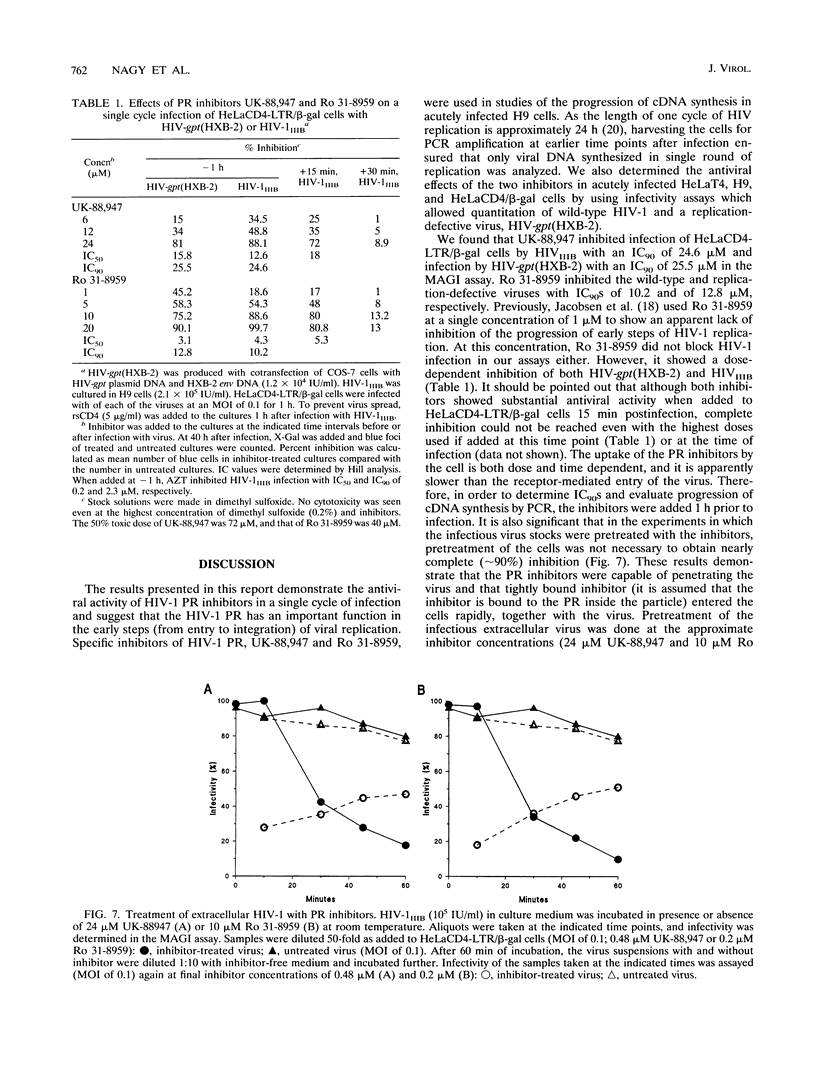
The antiviral activities of two substrate-based inhibitors of human immunodeficiency virus type 1 (HIV-1) protease, UK-88,947 and Ro 31-8959, were studied in acute infections. H9 and HeLaCD4-LTR/beta-gal cells were infected either with HIV-1IIIB or a replication-defective virus, HIV-gpt(HXB-2). Both inhibitors were capable of blocking early steps of HIV-1 replication if added to cells prior to infection. Partial inhibition was also obtained by addition of inhibitor at the time of or as late as 15 min after infection. The inhibitors were ineffective if added 30 min postinfection. The inhibitory effects were studied by cDNA analysis with PCR followed by Southern blot hybridization and by infectivity assays allowing quantitation of HIV-1 in a single cycle of replication. When UK-88,947-treated H9 cells were coinfected with HIV-1 and human T-cell leukemia virus type I only the replication of HIV-1 was inhibited, demonstrating viral specificity. Pretreating the infectious virus stocks with the inhibitors also prevented replication, indicating that the inhibitors block the action of the viral protease and not a cellular protease. A panel of primer sets was used to analyze cDNA from cell lysates by PCR amplification at 4 and 18 h postinfection. Four hours after infection, viral specific cDNA was detected with all of the four primer pairs used: R/U5, nef/U3, 5' gag, and long terminal repeat (LTR)/gag. However, after 18 h, only the R/U5 and nef/U3 primer pairs and not the 5' gag or LTR/gag primer pair were able to allow amplification of cDNA. The results suggest a crucial role of HIV-1 protease in the early phase of viral replication. Although it is not clear what early steps are affected by the protease, it is likely that the target is the NC protein, as referred from our previous reports of the in situ cleavage of the nucleocapsid (NC) protein by the viral protease inside lentiviral capsids. The results suggest that it is not the inhibition of initiation and progression of reverse transcription but the stability of full-size unintegrated cDNA which is affected in the presence of protease inhibitors. Alternatively, the cleavage of the NC protein may be required for the proper formation of preintegration complex and/or for its transport to the nucleus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert J., Fenyö E. M. Simple, sensitive, and specific detection of human immunodeficiency virus type 1 in clinical specimens by polymerase chain reaction with nested primers. J Clin Microbiol. 1990 Jul;28(7):1560–1564. doi: 10.1128/jcm.28.7.1560-1564.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboonian C., Dalgleish A., Bountiff L., Gross J., Oroszlan S., Rickett G., Smith-Burchnell C., Troke P., Merson J. HIV-1 proteinase is required for synthesis of pro-viral DNA. Biochem Biophys Res Commun. 1991 Aug 30;179(1):17–24. doi: 10.1016/0006-291x(91)91327-9. [DOI] [PubMed] [Google Scholar]
- Bowerman B., Brown P. O., Bishop J. M., Varmus H. E. A nucleoprotein complex mediates the integration of retroviral DNA. Genes Dev. 1989 Apr;3(4):469–478. doi: 10.1101/gad.3.4.469. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Damay P., Spahr P. F. Effect of rearrangements and duplications of the Cys-His motifs of Rous sarcoma virus nucleocapsid protein. J Virol. 1993 Feb;67(2):623–631. doi: 10.1128/jvi.67.2.623-631.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M. I., Sharova N., McDonald T. L., Pushkarskaya T., Tarpley W. G., Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciminale V., Pavlakis G. N., Derse D., Cunningham C. P., Felber B. K. Complex splicing in the human T-cell leukemia virus (HTLV) family of retroviruses: novel mRNAs and proteins produced by HTLV type I. J Virol. 1992 Mar;66(3):1737–1745. doi: 10.1128/jvi.66.3.1737-1745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P., Nagy K., Cheingsong-Popov R., Exley M., Weiss R. A. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. doi: 10.1126/science.6316502. [DOI] [PubMed] [Google Scholar]
- Debyser Z., Vandamme A. M., Pauwels R., Baba M., Desmyter J., De Clercq E. Kinetics of inhibition of endogenous human immunodeficiency virus type 1 reverse transcription by 2',3'-dideoxynucleoside 5'-triphosphate, tetrahydroimidazo-[4,5,1-jk][1,4]-benzodiazepin-2(1H)-thion e, and 1-[(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine derivatives. J Biol Chem. 1992 Jun 15;267(17):11769–11776. [PubMed] [Google Scholar]
- Dorfman T., Luban J., Goff S. P., Haseltine W. A., Göttlinger H. G. Mapping of functionally important residues of a cysteine-histidine box in the human immunodeficiency virus type 1 nucleocapsid protein. J Virol. 1993 Oct;67(10):6159–6169. doi: 10.1128/jvi.67.10.6159-6169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan N., Gavalchin J., Paul B., Wells K. H., Lane M. J., Poiesz B. J. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J Clin Microbiol. 1992 Apr;30(4):905–910. doi: 10.1128/jcm.30.4.905-910.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet C. M., Haseltine W. A. Determination of viral proteins present in the human immunodeficiency virus type 1 preintegration complex. J Virol. 1991 Apr;65(4):1910–1915. doi: 10.1128/jvi.65.4.1910-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R., Stålhanske P., von Gegerfelt A., Lind B., Aman P., Rassart E., Fenyö E. M. Biological characterization of infectious molecular clones derived from a human immunodeficiency virus type-1 isolate with rapid/high replicative capacity. Virology. 1991 Mar;181(1):55–61. doi: 10.1016/0042-6822(91)90469-r. [DOI] [PubMed] [Google Scholar]
- Gorelick R. J., Chabot D. J., Rein A., Henderson L. E., Arthur L. O. The two zinc fingers in the human immunodeficiency virus type 1 nucleocapsid protein are not functionally equivalent. J Virol. 1993 Jul;67(7):4027–4036. doi: 10.1128/jvi.67.7.4027-4036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett I. K., Geyer S. J., Hawthorne C. A., Ruta M., Epstein J. S. Kinetics of early HIV-1 gene expression in infected H9 cells assessed by PCR. Oncogene. 1991 Mar;6(3):491–493. [PubMed] [Google Scholar]
- Huff J. R. HIV protease: a novel chemotherapeutic target for AIDS. J Med Chem. 1991 Aug;34(8):2305–2314. doi: 10.1021/jm00112a001. [DOI] [PubMed] [Google Scholar]
- Jacobsen H., Ahlborn-Laake L., Gugel R., Mous J. Progression of early steps of human immunodeficiency virus type 1 replication in the presence of an inhibitor of viral protease. J Virol. 1992 Aug;66(8):5087–5091. doi: 10.1128/jvi.66.8.5087-5091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Zack J. A., Knigge M., Paul D. A., Kempf D. J., Norbeck D. W., Swanstrom R. Partial inhibition of the human immunodeficiency virus type 1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993 Jul;67(7):4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. Y., Byrn R., Groopman J., Baltimore D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J Virol. 1989 Sep;63(9):3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimpton J., Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol. 1992 Apr;66(4):2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapadat-Tapolsky M., De Rocquigny H., Van Gent D., Roques B., Plasterk R., Darlix J. L. Interactions between HIV-1 nucleocapsid protein and viral DNA may have important functions in the viral life cycle. Nucleic Acids Res. 1993 Feb 25;21(4):831–839. doi: 10.1093/nar/21.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M., Coffin J. M. Relationship of avian retrovirus DNA synthesis to integration in vitro. Mol Cell Biol. 1991 Mar;11(3):1419–1430. doi: 10.1128/mcb.11.3.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Putney S. D., Robinson H. L. Human immunodeficiency virus type 1 entry into T cells: more-rapid escape from an anti-V3 loop than from an antireceptor antibody. J Virol. 1992 Apr;66(4):2547–2550. doi: 10.1128/jvi.66.4.2547-2550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X. Y., Sakai K., Sinangil F., Golub E., Volsky D. J. Interaction of a noncytopathic human immunodeficiency virus type 1 (HIV-1) with target cells: efficient virus entry followed by delayed expression of its RNA and protein. Virology. 1990 May;176(1):184–194. doi: 10.1016/0042-6822(90)90243-k. [DOI] [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Meek T. D. Inhibitors of HIV-1 protease. J Enzyme Inhib. 1992;6(1):65–98. doi: 10.3109/14756369209041357. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature. 1990 Jan 4;343(6253):90–92. doi: 10.1038/343090a0. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Méric C., Goff S. P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989 Apr;63(4):1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Luftig R. B. Retroviral proteinases. Curr Top Microbiol Immunol. 1990;157:153–185. doi: 10.1007/978-3-642-75218-6_6. [DOI] [PubMed] [Google Scholar]
- Page K. A., Landau N. R., Littman D. R. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990 Nov;64(11):5270–5276. doi: 10.1128/jvi.64.11.5270-5276.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Rice W. G., Schaeffer C. A., Graham L., Bu M., McDougal J. S., Orloff S. L., Villinger F., Young M., Oroszlan S., Fesen M. R. The site of antiviral action of 3-nitrosobenzamide on the infectivity process of human immunodeficiency virus in human lymphocytes. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9721–9724. doi: 10.1073/pnas.90.20.9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. M., Copeland T. D., Oroszlan S. In situ processing of a retroviral nucleocapsid protein by the viral proteinase. Protein Eng. 1991 Aug;4(6):695–700. doi: 10.1093/protein/4.6.695. [DOI] [PubMed] [Google Scholar]
- Roberts M. M., Oroszlan S. The preparation and biochemical characterization of intact capsids of equine infectious anemia virus. Biochem Biophys Res Commun. 1989 Apr 28;160(2):486–494. doi: 10.1016/0006-291x(89)92459-5. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Tözsér J., Friedman D., Weber I. T., Bláha I., Oroszlan S. Studies on the substrate specificity of the proteinase of equine infectious anemia virus using oligopeptide substrates. Biochemistry. 1993 Apr 6;32(13):3347–3353. doi: 10.1021/bi00064a018. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Erickson J. W. Structure-based inhibitors of HIV-1 protease. Annu Rev Biochem. 1993;62:543–585. doi: 10.1146/annurev.bi.62.070193.002551. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]