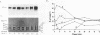Abstract
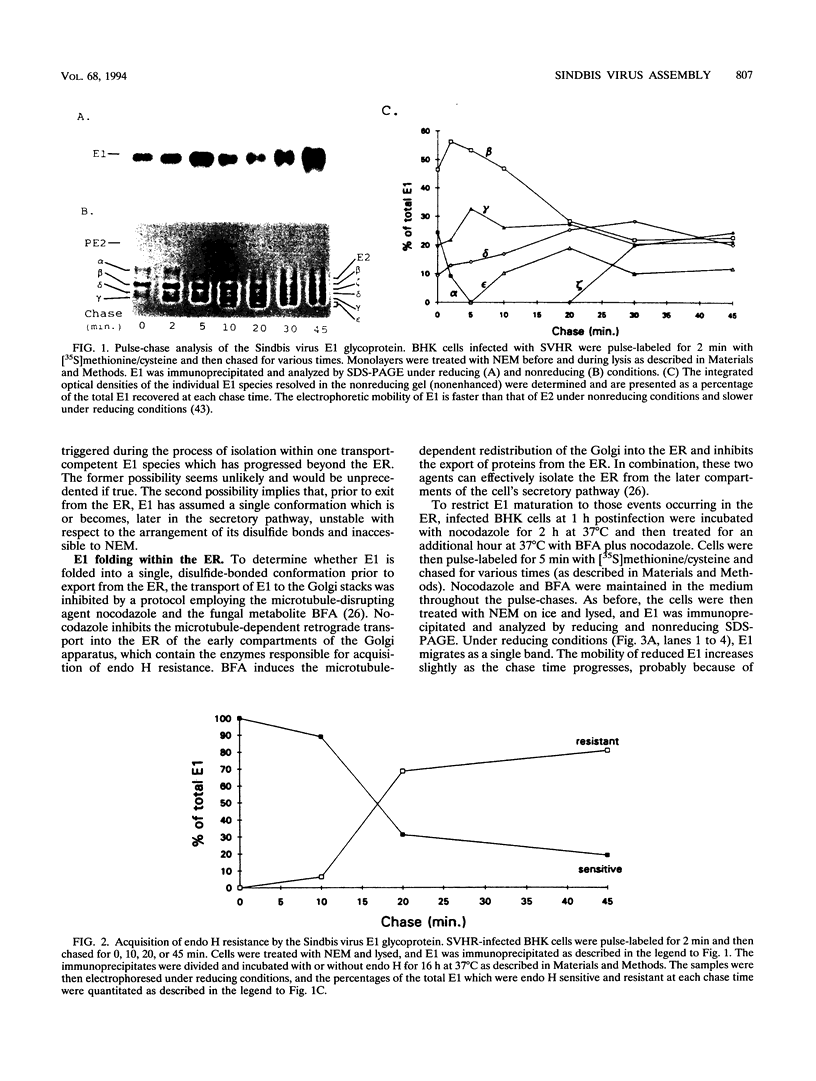
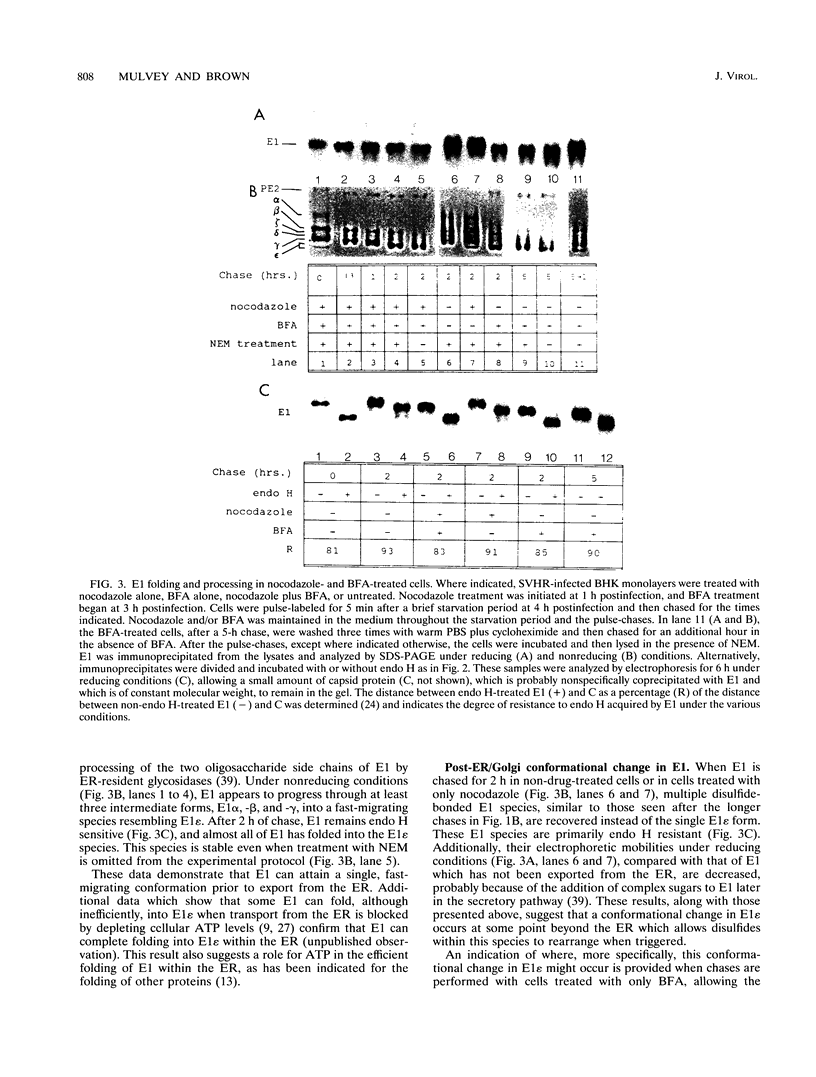
The rigidly ordered icosahedral lattice of the Sindbis virus envelope is composed of a host-derived membrane bilayer in which the viral glycoproteins E1 and E2 reside. E1-E1 interactions stabilized by intramolecular disulfide bridges play a significant role in maintaining the envelope's structural integrity (R. P. Anthony and D. T. Brown, J. Virol. 65:1187-1194, 1991; R. P. Anthony, A. M. Paredes, and D. T. Brown, Virology 190:330-336, 1992). We have examined the acquisition of disulfide bridges within E1 during its maturation. Prior to exit from the endoplasmic reticulum, E1 folds via at least three intermediates, differing in the number and/or arrangement of their disulfides, into a single, compact form. This E1 species remains stable with respect to its disulfides until late in the secretory pathway, when E1 attains a metastable conformation. At this point, when appropriately triggered, intramolecular thiol-disulfide exchange reactions within E1 can occur, resulting in the generation of alternative E1 species. This metastable nature of mature E1 may have important implications for the mechanism of virus disassembly during the initial stages of the infection process (B. Abell and D. T. Brown, J. Virol. 67:5496-5501, 1993).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell B. A., Brown D. T. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J Virol. 1993 Sep;67(9):5496–5501. doi: 10.1128/jvi.67.9.5496-5501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. P., Brown D. T. Protein-protein interactions in an alphavirus membrane. J Virol. 1991 Mar;65(3):1187–1194. doi: 10.1128/jvi.65.3.1187-1194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. P., Paredes A. M., Brown D. T. Disulfide bonds are essential for the stability of the Sindbis virus envelope. Virology. 1992 Sep;190(1):330–336. doi: 10.1016/0042-6822(92)91219-k. [DOI] [PubMed] [Google Scholar]
- Bonatti S., Migliaccio G., Blobel G., Walter P. Role of signal recognition particle in the membrane assembly of Sindbis viral glycoproteins. Eur J Biochem. 1984 May 2;140(3):499–502. doi: 10.1111/j.1432-1033.1984.tb08130.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty H., Wagner K. R., Helenius A. Folding of influenza hemagglutinin in the endoplasmic reticulum. J Cell Biol. 1991 Aug;114(3):401–411. doi: 10.1083/jcb.114.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Copeland C. S., Zimmer K. P., Wagner K. R., Healey G. A., Mellman I., Helenius A. Folding, trimerization, and transport are sequential events in the biogenesis of influenza virus hemagglutinin. Cell. 1988 Apr 22;53(2):197–209. doi: 10.1016/0092-8674(88)90381-9. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Disulfide bond formation in proteins. Methods Enzymol. 1984;107:305–329. doi: 10.1016/0076-6879(84)07021-x. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Lamb R. A., Rose J. K., Helenius A. Folding and assembly of viral membrane proteins. Virology. 1993 Apr;193(2):545–562. doi: 10.1006/viro.1993.1164. [DOI] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Moss B., Doms R. W. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J Virol. 1991 Apr;65(4):2047–2055. doi: 10.1128/jvi.65.4.2047-2055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Intracellular distribution of Sindbis virus membrane proteins in BHK-21 cells infected with wild-type virus and maturation-defective mutants. J Virol. 1980 Dec;36(3):775–786. doi: 10.1128/jvi.36.3.775-786.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn D. C., Meyer W. J., Mackenzie J. M., Jr, Johnston R. E. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interaction. J Virol. 1990 Aug;64(8):3643–3653. doi: 10.1128/jvi.64.8.3643-3653.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidwitz S., Polo J. M., Davis N. L., Johnston R. E. Differences in virion stability among Sindbis virus pathogenesis mutants. Virus Res. 1988 May;10(2-3):225–239. doi: 10.1016/0168-1702(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. The rubella virus E2 and E1 spike glycoproteins are targeted to the Golgi complex. J Cell Biol. 1993 Apr;121(2):269–281. doi: 10.1083/jcb.121.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Kim P. S., Bole D., Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992 Aug;118(3):541–549. doi: 10.1083/jcb.118.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipfer M. E., Brown D. T. Intracellular transport and processing of Sindbis virus glycoproteins. Virology. 1989 May;170(1):117–122. doi: 10.1016/0042-6822(89)90358-9. [DOI] [PubMed] [Google Scholar]
- Levy-Mintz P., Kielian M. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J Virol. 1991 Aug;65(8):4292–4300. doi: 10.1128/jvi.65.8.4292-4300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Liu N., Brown D. T. Phosphorylation and dephosphorylation events play critical roles in Sindbis virus maturation. Virology. 1993 Oct;196(2):703–711. doi: 10.1006/viro.1993.1527. [DOI] [PubMed] [Google Scholar]
- Liu N., Brown D. T. Transient translocation of the cytoplasmic (endo) domain of a type I membrane glycoprotein into cellular membranes. J Cell Biol. 1993 Feb;120(4):877–883. doi: 10.1083/jcb.120.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990 Oct;64(10):4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Johnston R. E. Structural rearrangement of infecting Sindbis virions at the cell surface: mapping of newly accessible epitopes. J Virol. 1993 Sep;67(9):5117–5125. doi: 10.1128/jvi.67.9.5117-5125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson T. S., Bamberger M. J., Lane M. D. Post-translational changes in tertiary and quaternary structure of the insulin proreceptor. Correlation with acquisition of function. J Biol Chem. 1988 May 25;263(15):7342–7351. [PubMed] [Google Scholar]
- Omar A., Koblet H. Semliki Forest virus particles containing only the E1 envelope glycoprotein are infectious and can induce cell-cell fusion. Virology. 1988 Sep;166(1):17–23. doi: 10.1016/0042-6822(88)90141-9. [DOI] [PubMed] [Google Scholar]
- Persson R., Pettersson R. F. Formation and intracellular transport of a heterodimeric viral spike protein complex. J Cell Biol. 1991 Jan;112(2):257–266. doi: 10.1083/jcb.112.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Strauss J. H. Association of sindbis virion glycoproteins and their precursors. J Mol Biol. 1982 Jan 15;154(2):325–348. doi: 10.1016/0022-2836(82)90067-5. [DOI] [PubMed] [Google Scholar]
- Scheefers H., Scheefers-Borchel U., Edwards J., Brown D. T. Distribution of virus structural proteins and protein-protein interactions in plasma membrane of baby hamster kidney cells infected with Sindbis or vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7277–7281. doi: 10.1073/pnas.77.12.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Mottet G., Kolakofsky D., Roux L. Addition of high-mannose sugars must precede disulfide bond formation for proper folding of Sendai virus glycoproteins. J Virol. 1989 Feb;63(2):892–900. doi: 10.1128/jvi.63.2.892-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg J. M., Garoff H. Membrane fusion process of Semliki Forest virus. I: Low pH-induced rearrangement in spike protein quaternary structure precedes virus penetration into cells. J Cell Biol. 1992 Jan;116(2):339–348. doi: 10.1083/jcb.116.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust C. J., Nicholas J. A., Fredin D., Dodd D. C., Brideau R. J., Levely M. E., Brown A. Monoclonal antibodies that cross-react with the E1 glycoprotein of different alphavirus serogroups: characterization including passive protection in vivo. Virus Res. 1989 Jun;13(2):101–112. doi: 10.1016/0168-1702(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Garoff H., Simons K. Formation of the Semliki Forest virus membrane glycoprotein complexes in the infected cell. J Gen Virol. 1980 Sep;50(1):111–123. doi: 10.1099/0022-1317-50-1-111. [DOI] [PubMed] [Google Scholar]
- de Curtis I., Simons K. Dissection of Semliki Forest virus glycoprotein delivery from the trans-Golgi network to the cell surface in permeabilized BHK cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8052–8056. doi: 10.1073/pnas.85.21.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A., Braakman I., Helenius A. Posttranslational folding of vesicular stomatitis virus G protein in the ER: involvement of noncovalent and covalent complexes. J Cell Biol. 1993 Feb;120(3):647–655. doi: 10.1083/jcb.120.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]