Abstract
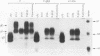
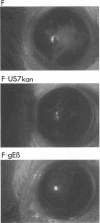
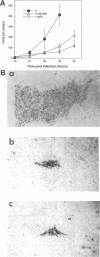
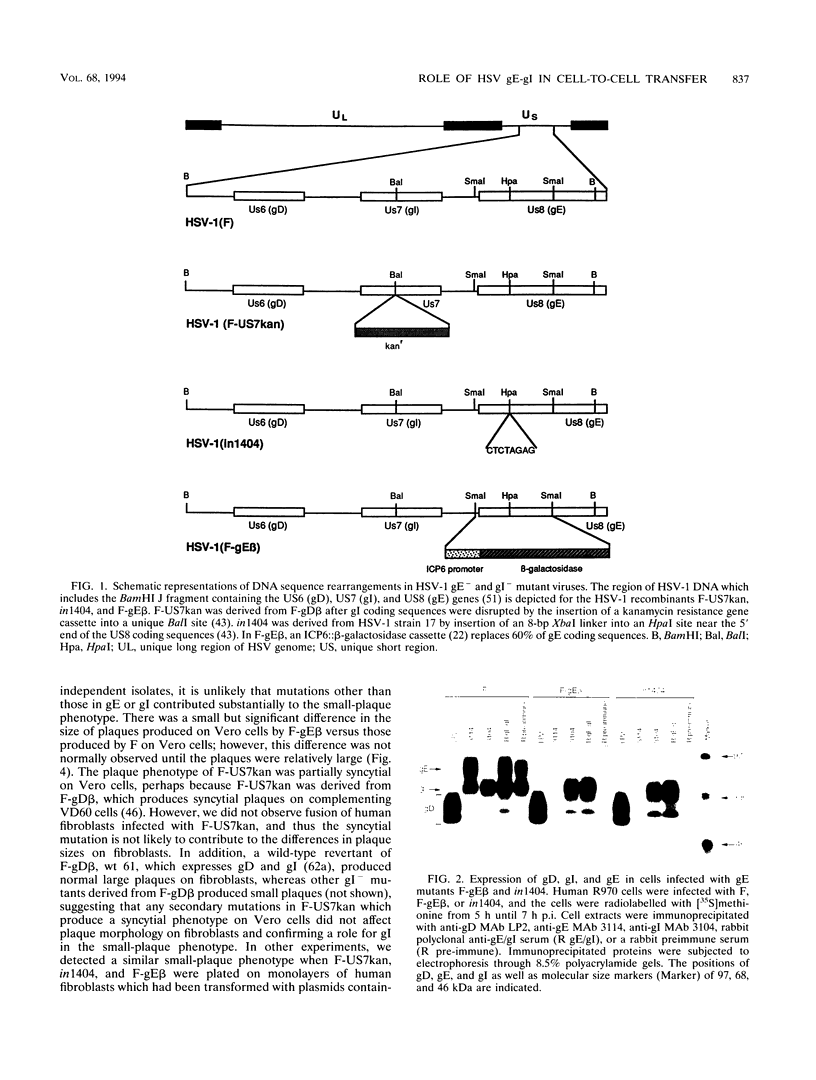
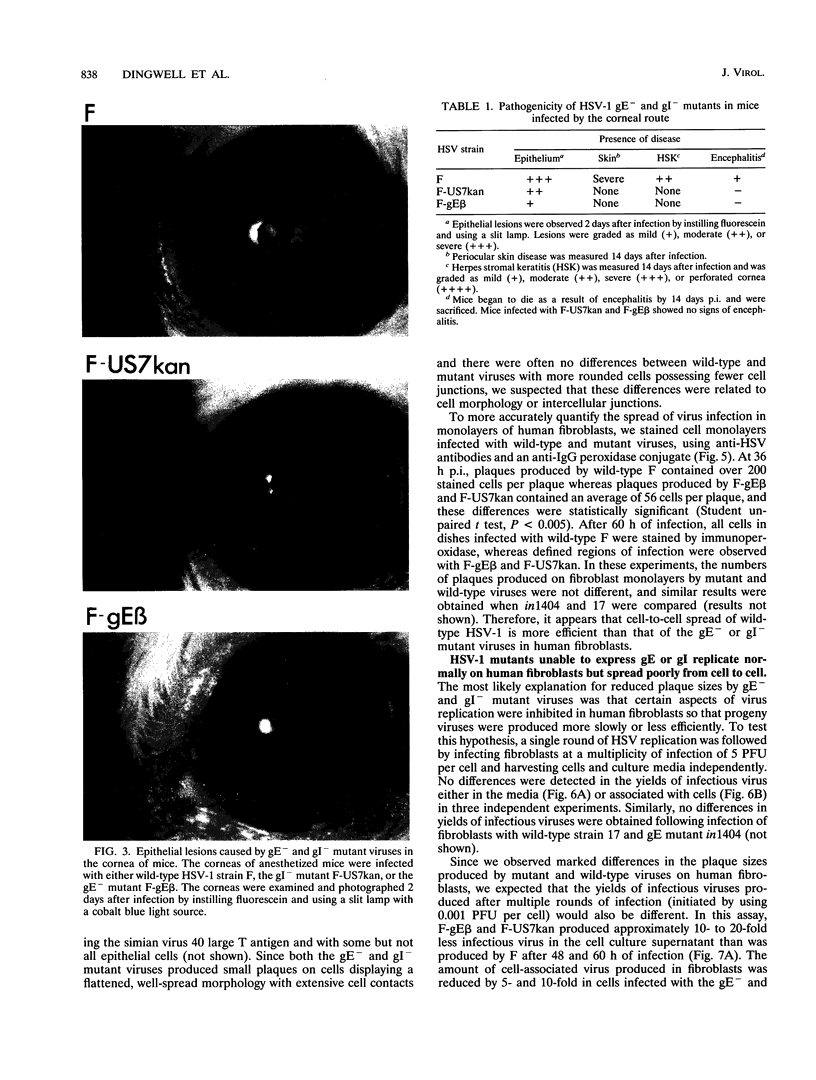
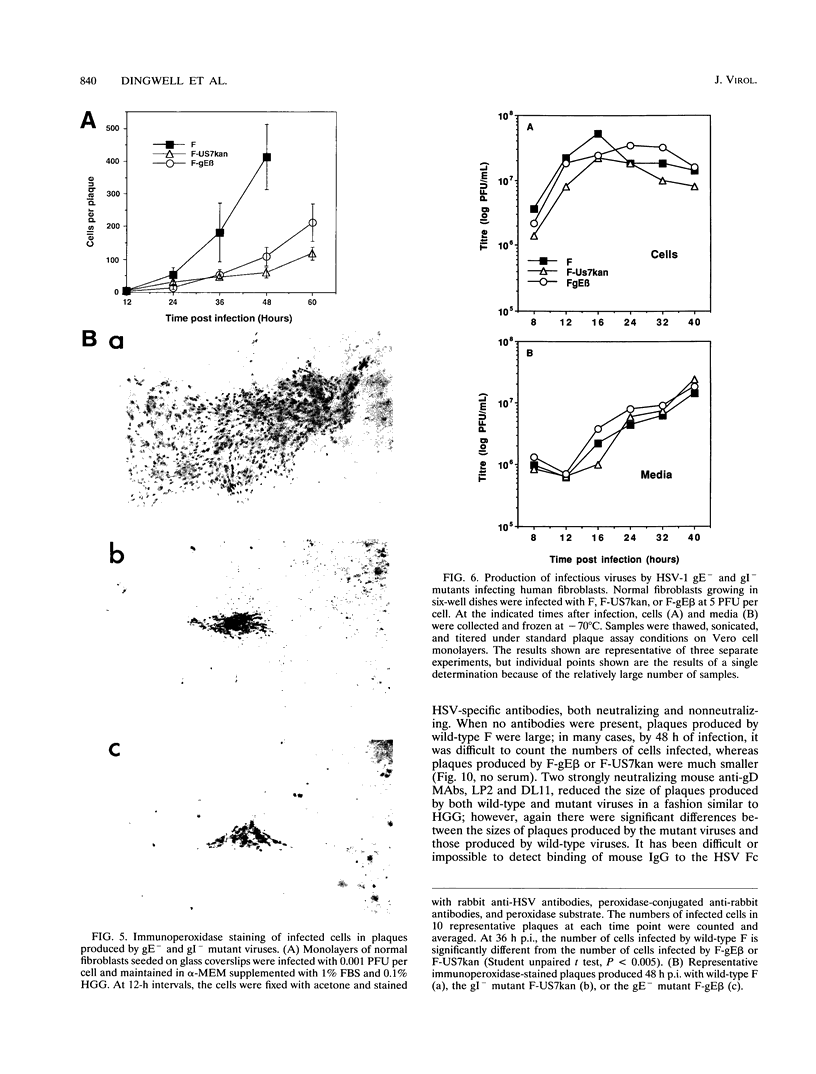
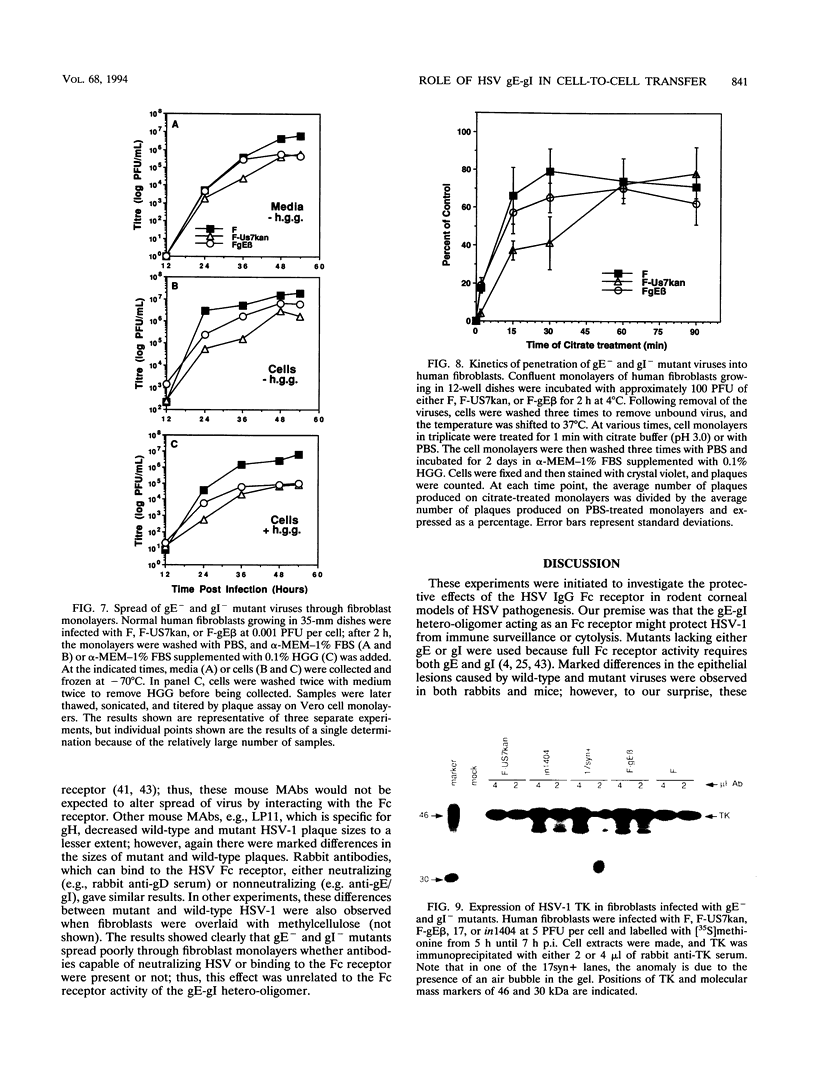
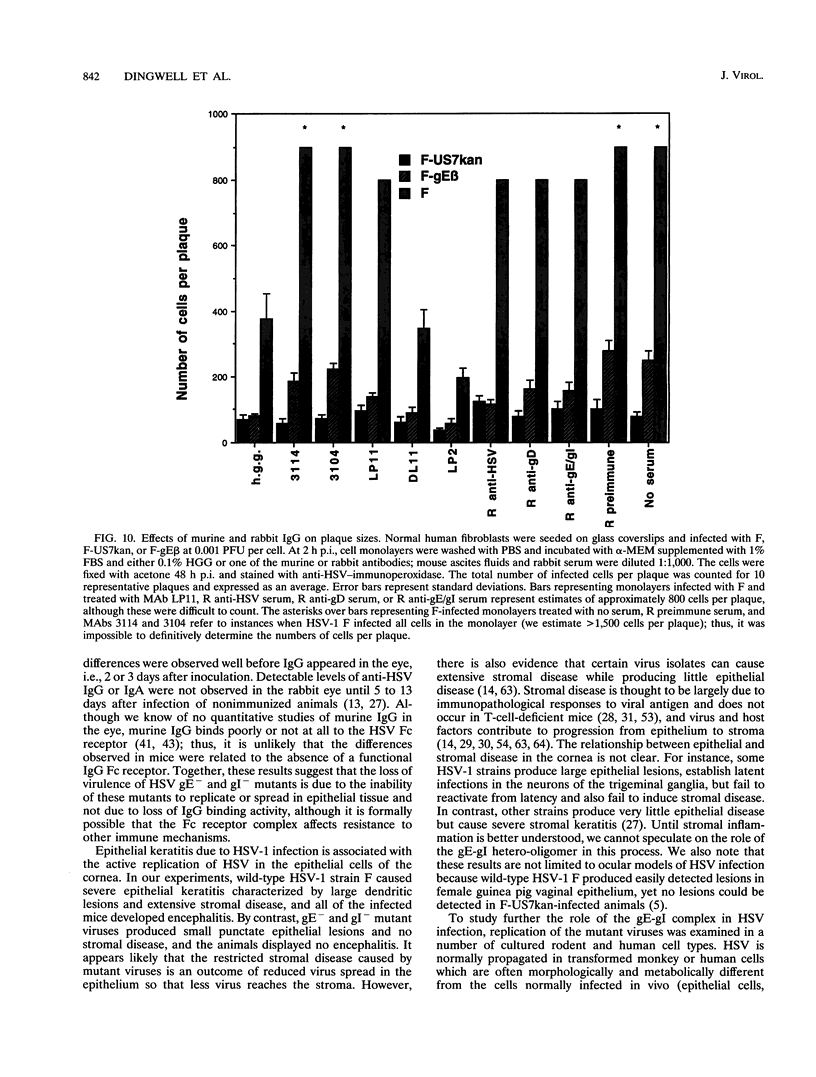
Herpes simplex virus (HSV) glycoproteins E and I (gE and gI) can act as a receptor for the Fc domain of immunoglobulin G (IgG). To examine the role of HSV IgG Fc receptor in viral pathogenesis, rabbits and mice were infected by the corneal route with HSV gE- or gI- mutants. Wild-type HSV-1 produced large dendritic lesions in the corneal epithelium and subsequent stromal disease leading to viral encephalitis, whereas gE- and gI- mutant viruses produced microscopic punctate or small dendritic lesions in the epithelium and no corneal disease or encephalitis. These differences were not related to the ability of the gE-gI oligomer to bind IgG because the differences were observed before the appearance of anti-HSV IgG and in mice, in which IgG binds to the Fc receptor poorly or not at all. Mutant viruses produced small plaques on monolayers of normal human fibroblasts and epithelial cells. Replication of gE- and gI- mutant viruses in human fibroblasts were normal, and the rates of entry of mutant and wild-type viruses into fibroblasts were similar; however, spread of gE- and gI- mutant viruses from cell to cell was significantly slower than that of wild-type HSV-1. In experiments in which fibroblast monolayers were infected with low multiplicities of virus and multiple rounds of infection occurred, the presence of neutralizing antibodies in the culture medium caused the yields of mutant viruses to drop dramatically, whereas there was a lesser effect on the production of wild-type HSV. It appears that cell-to-cell transmission of wild-type HSV-1 occurs by at least two mechanisms: (i) release of virus from cells and entry of extracellular virus into a neighboring cell and (ii) transfer of virus across cell junctions in a manner resistant to neutralizing antibodies. Our results suggest that gE- and gI- mutants are defective in the latter mechanism of spread, suggesting the possibility that the gE-gI complex facilitates virus transfer across cell junctions, a mode of spread which may predominate in some tissues. It is ironic that the gE-gI complex, usually considered an IgG Fc receptor, may, through its ability to mediate cell-to-cell spread, actually protect HSV from IgG in a manner different than previously thought.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993 Mar;67(3):1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991 Feb;65(2):938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- Cai W. H., Gu B., Person S. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J Virol. 1988 Aug;62(8):2596–2604. doi: 10.1128/jvi.62.8.2596-2604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Arsenakis M., Farabegoli F., Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988 Jan;62(1):159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card J. P., Whealy M. E., Robbins A. K., Enquist L. W. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J Virol. 1992 May;66(5):3032–3041. doi: 10.1128/jvi.66.5.3032-3041.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C., Easty D. L. Experimental ulcerative herpetic keratitis. I. Systemic immune responses and resistance to corneal infection. Br J Ophthalmol. 1981 Feb;65(2):77–81. doi: 10.1136/bjo.65.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centifanto-Fitzgerald Y. M., Yamaguchi T., Kaufman H. E., Tognon M., Roizman B. Ocular disease pattern induced by herpes simplex virus is genetically determined by a specific region of viral DNA. J Exp Med. 1982 Feb 1;155(2):475–489. doi: 10.1084/jem.155.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler K. W., Veltri R. W. In vitro neutralization of HSV-2: inhibition by binding of normal IgG and purified Fc to virion Fc receptor (FcR). J Med Virol. 1984;13(3):251–259. doi: 10.1002/jmv.1890130307. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Hastings J. C., Cohen G. H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987 Dec;3(6):423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Forrester A., Farrell H., Wilkinson G., Kaye J., Davis-Poynter N., Minson T. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992 Jan;66(1):341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I., Friedman H. M. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989 Nov;63(11):4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Fuller A. O., Santos R. E., Spear P. G. Neutralizing antibodies specific for glycoprotein H of herpes simplex virus permit viral attachment to cells but prevent penetration. J Virol. 1989 Aug;63(8):3435–3443. doi: 10.1128/jvi.63.8.3435-3443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988 Aug;62(8):2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gompels U., Minson A. The properties and sequence of glycoprotein H of herpes simplex virus type 1. Virology. 1986 Sep;153(2):230–247. doi: 10.1016/0042-6822(86)90026-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Lulitanond V., Johnson D. C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990 Aug;177(2):437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks R. L., Epstein R. J., Tumpey T. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Invest Ophthalmol Vis Sci. 1989 Jan;30(1):105–115. [PubMed] [Google Scholar]
- Hendricks R. L., Janowicz M., Tumpey T. M. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992 Apr 15;148(8):2522–2529. [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res. 1991;10 (Suppl):47–53. doi: 10.3109/02713689109020357. [DOI] [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990 Oct;31(10):1929–1939. [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M., Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992 Nov 1;149(9):3023–3028. [PubMed] [Google Scholar]
- Herold B. C., WuDunn D., Soltys N., Spear P. G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991 Mar;65(3):1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Dorney D. J., Gage P. J., Holland T. C., Cai W., Person S., Levine M., Glorioso J. C. Identification of mar mutations in herpes simplex virus type 1 glycoprotein B which alter antigenic structure and function in virus penetration. J Virol. 1989 Feb;63(2):730–738. doi: 10.1128/jvi.63.2.730-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highlander S. L., Sutherland S. L., Gage P. J., Johnson D. C., Levine M., Glorioso J. C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987 Nov;61(11):3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt G. D., Krivan H. C., Gasic G. J., Ginsburg V. Antistasin, an inhibitor of coagulation and metastasis, binds to sulfatide (Gal(3-SO4) beta 1-1Cer) and has a sequence homology with other proteins that bind sulfated glycoconjugates. J Biol Chem. 1989 Jul 25;264(21):12138–12140. [PubMed] [Google Scholar]
- Huszar D., Beharry S., Bacchetti S. Herpes simplex virus-induced ribonucleotide reductase: development of antibodies specific for the enzyme. J Gen Virol. 1983 Jun;64(Pt 6):1327–1335. doi: 10.1099/0022-1317-64-6-1327. [DOI] [PubMed] [Google Scholar]
- Hutchinson L., Browne H., Wargent V., Davis-Poynter N., Primorac S., Goldsmith K., Minson A. C., Johnson D. C. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J Virol. 1992 Apr;66(4):2240–2250. doi: 10.1128/jvi.66.4.2240-2250.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P. J., Myhre E. B., Blomberg J. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J Virol. 1985 Nov;56(2):489–494. doi: 10.1128/jvi.56.2.489-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Burke R. L., Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990 Jun;64(6):2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Ligas M. W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988 Dec;62(12):4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. M., Spear P. G. Herpes simplex virus glycoprotein D mediates interference with herpes simplex virus infection. J Virol. 1989 Feb;63(2):819–827. doi: 10.1128/jvi.63.2.819-827.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V., Jackson W., Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992 Jun;66(6):3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Clustering of genes dispensable for growth in culture in the S component of the HSV-1 genome. Science. 1987 May 1;236(4801):573–576. doi: 10.1126/science.3033823. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Mavromara-Nazos P., Sears A. E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988 Jan;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- Metcalf J. F., Hamilton D. S., Reichert R. W. Herpetic keratitis in athymic (nude) mice. Infect Immun. 1979 Dec;26(3):1164–1171. doi: 10.1128/iai.26.3.1164-1171.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. F., Michaelis B. A. Herpetic keratitis in inbred mice. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1222–1225. [PubMed] [Google Scholar]
- Minson A. C., Hodgman T. C., Digard P., Hancock D. C., Bell S. E., Buckmaster E. A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986 Jun;67(Pt 6):1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- Navarro D., Paz P., Pereira L. Domains of herpes simplex virus I glycoprotein B that function in virus penetration, cell-to-cell spread, and cell fusion. Virology. 1992 Jan;186(1):99–112. doi: 10.1016/0042-6822(92)90064-v. [DOI] [PubMed] [Google Scholar]
- Neidhardt H., Schröder C. H., Kaerner H. C. Herpes simplex virus type 1 glycoprotein E is not indispensable for viral infectivity. J Virol. 1987 Feb;61(2):600–603. doi: 10.1128/jvi.61.2.600-603.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters B., Pol J., Gielkens A., Moormann R. Envelope glycoprotein gp50 of pseudorabies virus is essential for virus entry but is not required for viral spread in mice. J Virol. 1993 Jan;67(1):170–177. doi: 10.1128/jvi.67.1.170-177.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcáni J., Herget U., Kaerner H. C. Spread of herpes simplex virus (HSV) strains SC16, ANG, ANGpath and its glyC minus and GlyE minus mutants in DBA-2 mice. Acta Virol. 1990 Aug;34(4):305–320. [PubMed] [Google Scholar]
- Roop C., Hutchinson L., Johnson D. C. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J Virol. 1993 Apr;67(4):2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., McGwire B. S., Roizman B. Infection of polarized MDCK cells with herpes simplex virus 1: two asymmetrically distributed cell receptors interact with different viral proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5087–5091. doi: 10.1073/pnas.88.12.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. R., Fong B. S., Leung W. C. Construction of a double-jointed herpes simplex viral DNA molecule: inverted repeats are required for segment inversion, and direct repeats promote deletions. Virology. 1981 Aug;113(1):345–362. doi: 10.1016/0042-6822(81)90161-6. [DOI] [PubMed] [Google Scholar]
- Smiley J. R., Johnson D. C., Pizer L. I., Everett R. D. The ICP4 binding sites in the herpes simplex virus type 1 glycoprotein D (gD) promoter are not essential for efficient gD transcription during virus infection. J Virol. 1992 Feb;66(2):623–631. doi: 10.1128/jvi.66.2.623-631.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulting R. D., Kindle J. C., Nahmias A. J. Patterns of herpes simplex keratitis in inbred mice. Invest Ophthalmol Vis Sci. 1985 Oct;26(10):1360–1367. [PubMed] [Google Scholar]
- Wander A. H., Centifanto Y. M., Kaufman H. E. Strain specificity of clinical isolates of herpes simplex virus. Arch Ophthalmol. 1980 Aug;98(8):1458–1461. doi: 10.1001/archopht.1980.01020040310020. [DOI] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- Zsak L., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gI of pseudorabies virus promotes cell fusion and virus spread via direct cell-to-cell transmission. J Virol. 1992 Apr;66(4):2316–2325. doi: 10.1128/jvi.66.4.2316-2325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F. A., Mettenleiter T. C., Schreurs C., Sugg N., Ben-Porat T. Complex between glycoproteins gI and gp63 of pseudorabies virus: its effect on virus replication. J Virol. 1988 Dec;62(12):4622–4626. doi: 10.1128/jvi.62.12.4622-4626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]