Abstract
The 3’-to-5’ exonucleolytic decay and processing of a variety of RNAs is an essential feature of RNA metabolism in all cells. The 3’-5’ exonucleases, and in particular the exosome, are involved in a large number of pathways from 3’ processing of rRNA, snRNA and snoRNA, to decay of mRNAs and mRNA surveillance. The potent enzymes performing these reactions are regulated to prevent processing of inappropriate substrates while mature RNA molecules exhibit several attributes that enable them to evade 3’-5’ attack. How does an enzyme perform such selective activities on different substrates? The goal of this review is to provide an overview and perspectives of available data on the underlying principles for the recognition of RNA substrates by 3’-to-5’ exonucleases.
Introduction
Exoribonuclease activities play major roles in both prokaryotic and eukaryotic cells. First, exonucleases are vital for many pathways of RNA decay. RNA levels are determined not only by their rate of synthesis, but also by the rate of degradation. Thus, RNA turnover rates are an integral component of the control of gene expression. Importantly, 3’-to-5’ exonucleases (3’ exos) play both general and regulated roles in RNA decay. Second, aberrant RNA molecules are inevitably generated during transcription and processing. Several 3’ exos are essential to the removal of these aberrant transcripts from the cell. Finally, transcription does not terminate precisely at the 3’ end of a transcript, thus 3’ processing is essential to the maturation of both mRNAs and non-coding RNAs. 3’ exos have been implicated in many such processing events.
Not surprisingly, given the destructive activity of these enzymes, they experience various regulatory restrictions. Most will recognize only single-stranded 3’ RNA ends, others have sequence specificity, and often stimulatory co-factors are closely associated with the core enzymatic activity. In this review, we will discuss the various characterized 3’-to-5’ exonuclease activities, their substrate spec ificities and regulation. Given that the exosome, a large complex of exoribonucleases present in archaea and eukaryotes, is perhaps the most prolific 3’-to-5’ exonuclease activity known, we will devote a significant proportion of our discussion to this enzyme complex.
Roles of 3’ exos in eukaryotes
In all three kingdoms of life, the processing and decay of RNA by 3’ exos is an essential pathway. The functions of 3’ exos are surprisingly well conserved, although the factors involved are significantly more complex in eukaryotes.
RNA processing by 3’ exos
In eukaryotic cells, the vast majority of 3’ exo activity is contributed by the exosome, a complex of nine core and several auxiliary components. Since transcription termination does not occur precisely at the 3’ end of mature transcripts [53,61,63], this activity contributes to the 3’ end trimming/processing of several classes of RNAs in the nucleus. Deletion or mutation of exosome components leads to accumulation of transcripts with extended 3’ ends including 5.8S rRNA, snRNAs (e.g. U4 and U5) and snoRNAs (e.g. U14, U18, U24 that are excised from larger precursors) [1,2,68] Other 3’ exos may also contribute to the 3’ end trimming of 5S, U4 and MRP RNAs [81]. Interestingly, the exosome also contributes to an alternative pathway of 3’ end formation for mRNAs [31,77]. Finally, the exosome contributes directly or indirectly to a variety of RNA processing events. In the pathway of rRNA maturation, for example, exosome mutations directly lead to elevated levels of the 5’ external transcribed spacer region and indirectly to the reduction of cleavage efficiency at a variety of steps in the processing of the large ribosomal RNAs [1,2].
Likewise, in prokaryotes, 3’exos have been shown to play a role in processing of small stable RNAs, and rRNA. In Pseudomonas syringae, RNase R is involved in 3’ trimming of the 16S and 5S rRNAs [62] whilst in E. coli, 3’ exos, particularly RNase T and RNase PH, are essential for 3’ maturation of tRNA and other small RNAs [41,42].
Finally, a 3’ exo, 3’hExo, has recently been found associated with histone mRNAs. 3’hExo was initially thought to be involved in degradation of these transcripts [18], but it now seems more likely that it is required for cytoplasmic 3’ trimming [56].
3’ exos and mRNA degradation
In eukaryotic cells, the degradation of most mRNAs starts with deadenylation, which is performed by a variety of poly(A)-specific 3’ exos [25]. The mRNA body undergoes degradation either by the exosome (3′-to-5′ pathway) or by cleavage of the 5′ cap followed by 5′-to-3′ degradation by the Xrn1p exoribonuclease (5′-to-3′ pathway) [25]. In addition to its contribution to general mRNA stability, the exosome also contributes to regulated mRNA decay mediated by AU-rich elements [12,55] as well as to quality control of gene expression by degrading defective transcripts, such as mRNAs that have premature translation termination codons [75], or those which lack termination codons altogether [79]. The exosome is also involved in the degradation of the extensive array of cryptic nuclear RNA polymerase II transcripts that have recently been described [50]. Finally, other 3’-to-5’ exonucleases also contribute to specialized mRNA decay mechanisms. For example, the interferon-induced ISG20 3’exo has anti-viral activity [21].
In prokaryotic cells, 3’ exo activity is just as important to mRNA decay. A complex termed the degradosome is integral to the process [10]. The degradosome comprises an endonuclease (RNase E), a helicase (RhlB), enolase, and a 3’ exo (polynucleotide hosphorylase, PNPase). Decay usually initiates with endonucleolytic cleavage, followed by polyadenylation of mRNA fragments by poly(A) polymerase. These fragments are then substrates for 3’-to-5’ decay by PNPase and other 3’ exos [10,13].
The exosome and RNA interference
There is growing evidence that 3’-to-5’ exonucleases may also play an important and complex role in RNA interference pathways. First, the exosome has been implicated as a means to degrade the RNA fragments generated by RNAi-mediated cleavage [58]. Second, it has been suggested that 3′hExo [18] and its putative Caenorhabditis elegans homologue, ERI-1 [35], might play a role in down-regulation of RNA interference by degrading small interfering RNAs (siRNAs) since ERI-1 was identified by genome-wide scanning for mutants of C. elegans with enhanced RNA interference. Third, ERI-1 may play a positive role in the RNAi response by interacting with Dicer where it assists in the accumulation of several endogenous siRNAs and regulates the response to exogenous double-stranded RNAs [19]. In this model, ERI-1 binds to short stem-loops of endogenous RNAs and removes unpaired 3′ nucleotides, generating the structure required for synthesis of double-stranded RNA species which can be cleaved by Dicer to initiate the RNA interference cascade [19].
Classes of 3’-5’ exonucleases
3’exos cleave phosphodiester bonds through either a hydrolytic or a phosphorylytic mechanism resulting in production of nucleotide monophosphates or nucleotide diphosphates, respectively. There are four major classes of characterized 3’ exos: the RNR, DEDD, and PDX superfamilies all have members in eukaryotic, archaeal and bacterial kingdoms, whilst the RRP4 family is exclusive to eukaryotes and archaea [92].
RNR Superfamily
The RNAseR/RNAse II 3’ exos are non-specific, highly processive hydrolytic enzymes that bear three putative OB-fold type RNA-binding domains (one S1 domain and two cold-shock domains). Both enzymes have been implicated in decay of bacterial mRNAs. The bacterial RNase R is somewhat unique among 3’exos in that it can degrade structured RNAs on its own, provided that there is a 3’ single-stranded extension of more than seven nucleotides [83]. RNase II in contrast, can only degrade single-stranded RNAs [15].
In eukaryotes, the RNR superfamily is represented by Rrp44/Dis3, the catalytic component of the yeast exosome [20,44], and by Dss1, the active subunit of the yeast mitochondrial degradosome [48].
DEDD Superfamily
These hydrolytic enzymes are named for the four invariant amino acids required for activity and are represented by RNase T, RNase D and oligoribonuclease (orn) in prokaryotes. The bacterial RNases T and D contribute to 3’ maturation of several small stable RNAs [41,42] while orn is required for recycling of short oligonucleotide fragments 2-7nt long generated by other 3’ exo activities [27]. RNase T differs from other 3’ exos in that it can recognize very short single stranded regions of only 1–2 nucleotides [93]. This makes it the major processing enzyme for several small stable RNAs in E. coli.
In eukaryotes there are several different DEDD superfamily 3’ exos with various functions. The Rex proteins and Rrp6/PM-Scl100, like bacterial RNase T, are involved in 3’ maturation events, such as those of 5S rRNA, U4 snRNA and RNase P RNAs [8,80,81]. Rrp6/PMScl-100 is in fact an essential catalytic component of the nuclear exosome and is therefore involved in a wide range of activities including nuclear mRNA surveillance. Several eukaryotic poly(A)-specific exoribonucleases involved in mRNA decay, namely Ccr4, Caf1/Pop2, PARN, and Pan2, are also DEDD family members [54]. Two other DEDD exos, 3’hExo/ERI-1, the 3’ exo involved in histone mRNA decay and ISG20, an antiviral 3’ exo, have more specialized functions [21,89].
PDX Superfamily
These 3’ exos exhibit phosphate-dependent activity and are typified by the bacterial PNPase and RNase PH proteins [74]. Bacterial PNPase is a processive enzyme containing two RNase PH domains and is an essential component of the RNA degradosome [10]. The human PNPase has been implicated in decay of the c-myc mRNA as well as being an early response gene for the interferon pathway [65,66]. RNase PH proteins form a hexameric ring structure that is critical for their function. Bacterial RNAse PH is involved in distributive 3’ processing of tRNAs [33] whilst in eukaryotes and archaea, RNase PH proteins are integral components of the exosome core [74] and are therefore involved in a variety of processing and decay mechanisms.
Interestingly, although Rrp41 of the archaeal exosome is catalytic [45], in eukaryotes the PH domain proteins of the exosome appear to lack catalytic activity as the products of RNA degradation by the reconstituted yeast exosome are exclusively nucleotide monophosphates which would only be produced by hydrolytic activity [20,44]. In addition, both the human and yeast exosome cores lack any catalytic activity [20,44]. Thus in the exosome the PH domain proteins appear to serve an alternative role, perhaps contributing to enzyme structure, regulation and/or substrate recognition/binding.
The exosome
In eukaryotes, and archaea, a large proportion of processing and decay events requiring 3’-to-5’ exonuclease activity are performed by a complex of proteins known as the exosome. This intriguing complex contains a wide variety of 3’ exos, some of which are catalytic, and others that make structural contributions.
The core exosome, in both archaea and eukaryotes, consists of a six-member ring of RNase PH domain proteins. Archaea have three copies of the Rrp41/Rrp42 heterodimer, while eukaryotes have six different PH domain proteins (Rrp41, Rrp42, Rrp43/OIP2, Rrp45/PM-Scl75, Rrp46, Mtr3). Although the structures of the core archaeal and eukaryotic exosomes are very similar, their catalytic mechanism appears very different. In archaea, and Arabidopsis Rrp41 contributes catalytic activity [11,47], while for the yeast and human exosomes no detectable phosphorylytic activity could be observed, suggesting that the core plays more of a structural role [20,44]. In fact, catalytic activity in the yeast exosome is contributed by an RNR family member – Dis3/Rrp44 [20,44].
Several additional proteins associate with the core exosome and either provide catalytic capabilities or are involved in substrate recognition or modification. Accessory proteins of the exosome include three Rrp4 family members in eukaryotes (Rrp4, Rrp40 and Csl4), as well as the RNase D homolog Rrp6/PM-Scl100 in the nuclear exosome.
In eukaryotes, there are at least two distinct exosome complexes, one in the nucleus and the other in the cytoplasm. It is possible that smaller sub-complexes also exist in these compartments [28]. The nuclear exosome is distinguished by the presence of Rrp6/PM-Scl100 which is involved in degradation and processing of numerous nuclear RNA substrates. Also, in the nucleus the exosome is able to associate with a protein complex known as TRAMP. TRAMP comprises Trf4 (a poly(A) polymerase), Air1 (a zinc knuckle protein), and Mtr4 (an RNA helicase) and is involved in recognition and polyadenylation of a number of exosome substrates. In contrast, the cytoplasmic exosome interacts with the Ski complex which comprises Ski2 (an RNA helicase), Ski3 (a tetratricopeptide repeat protein) and Ski8 (a WD40 repeat protein), as well as the GTPase Ski7 [4,85]. The cytoplasmic exosome is an important mRNA decay and surveillance enzyme.
Substrate Preferences
RNA is a unique enzyme substrate in that each transcript is different, but must be recognized by the same set of enzymes for 3’-to-5’ processing and/or decay. Non-specific exoribonucleases must be regulated to prevent uncontrolled decay of all RNA molecules, whilst the specific enzymes must distinguish features such as secondary structure, or particular sequence motifs on appropriate RNA substrates. Substrates for 3’exos range from poly(A) to highly structured RNAs such as tRNA and rRNA. Moreover, the 3’ exos are required to exhibit both distributive and processive activities to facilitate either 3’ end trimming of short stretches or complete degradation of selected RNAs. In some cases, the 3’exo can act alone to process the substrate, but for the majority of cases, a helicase or other cofactor is required to direct the RNA into the active site of the enzyme. In the case of the exosome, an entire complex of proteins is required for recognition and processing or decay of substrate RNAs.
Degrading ssRNA
3’ exos are involved in the processing and decay of a wide range of substrates. Interestingly, different enzymes can often act interchangeably suggesting there are common preferences. One basic feature of many 3’ exo substrates is the presence of a 3’ single-stranded extension. Most mature non-coding RNAs have structured 3’ ends, whilst mature mRNAs are protected by association of PABP with the 3’ poly(A) tail, allowing the 3’UTR sequence to be unconstrained. Thus, a preference for single-stranded 3’ ends prevents non-specific 3’ exo activity from digesting inappropriate substrates. This affinity for unstructured RNA can be explained in part by examining the available protein structural data, which indicates that for several of these exonucleases the active site is positioned such that only unstructured RNA can enter.
The crystal structure of E. coli RNase II reveals a clamp-like arrangement of three RNA binding domains through which the ssRNA substrate is threaded into a narrow channel leading to the active site [24,94] (Figure 1a). The clamp is too small to allow access for dsRNA, thus explaining the enzyme’s inability to degrade structured RNA. This is also consistent with the role of RNase II in degrading poly(A) tails, particularly those appended to the 23S rRNA [51]. Moreover, the inability of RNase II to degrade structured RNA appears to allow it to stabilize some mRNAs by removing oligo(A) tails, thereby preventing access of other exonucleases such as PNPase [52].
Figure 1. Exonuclease structure determines substrate preference.

(a) RNase II – The S1 and Cold Shock Domains (CSD) are situated such that double stranded RNA cannot enter the narrow channel leading to the active site. This model is based on crystal structure of E. coli RNAse II
(b) RNase R – The S1 and CSD regions are positioned to allow double-stranded RNA to feed into the channel leading to the active site. There are no structural data available for RNase R, thus this model awaits experimental confirmation.
(c) Archaeal Exosome – Three views are shown. The RNAse PH domain proteins Rrp41 and Rrp42 form a hexameric ring structure with Rrp4 bridging each Rrp41 and Rrp42 dimer. The RNA feeds through the narrow central hole into the active site of Rrp41. Double stranded RNA cannot enter this channel.
(d) Eukaryotic Exosome – The six RNase PH domain proteins form a hexameric ring with Rrp4, Csl4 and Rrp40 bridging the dimers in a similar fashion as seen for the archaeal exosome. This nine-subunit exosome appears to play a structural role as it has no catalytic activity in vitro. The catalytic subunit, Rrp44 is depicted in the side views with two possible routes for entry of the RNA substrate into its active site.
The archaeal exosome core of six RNAse PH proteins forms a ring with a central channel. This structure is similar to that of prokaryotic PNPase. The structural data suggests that RNA must reach the active site on Rrp41 by threading through the narrow central channel, thus restricting substrates to unstructured RNAs [46] (Figure 1b). In the eukaryotic exosome (Figure 1d) the location of the active site, at least in the yeast exosome, lies outside the exosome core, within the Dis3/Rrp44 protein [20,44]. Recent EM data [84] suggest that the RNA may enter the Rrp44 active site by a different route depending on its identity (Figure 1d). RNAs with substantial single stranded 3’ ends would be able to thread through the exosome central channel and feed into the Rrp44 active site. However, RNAs with more secondary structure would likely favor direct entry into Rrp44 [84].
Degrading structured RNA
Turnover of structured RNAs presents a different problem, in that the structure must be unwound before cleavage can occur. RNase R is able to degrade structured RNA in the absence of other factors [83], and this ability appears to be conserved in the yeast RNR family member, Dis3/Rrp44 [44]. However, all other characterized 3’exos require assistance from other factors to degrade structured substrates.
E. coli RNase R is able to recognize and rapidly degrade structured RNAs providing there is a single-stranded 3’ overhang [83]. Based on the structure of the closely related RNase II, it has been proposed that instead of acting as a clamp, the RNA binding domains of RNase R funnel the structured RNA towards the active site [83] (Figure 1c). The channel itself is still only wide enough to accommodate ssRNA, explaining the requirement for an unpaired region of at least 7 nt and preferentially more than 10 nt. It seems that perhaps as nucleotides are removed, the RNA is pulled into the channel and this somehow forces the unwinding of the duplex RNA. More structural data are required to fully understand how RNAse R catalysis functions.
Other 3’exos that exhibit activity on structured RNAs are dependent on their association with RNA helicases for this function. The E. coli PNPase is essentially inactive on highly structured RNAs in vitro, but exists in vivo in complex with three other proteins, the RhlB helicase, enolase and RNase E, an endonuclease which also acts as a scaffold [16]. Together these proteins form the degradosome which can processively degrade a wide variety of RNA substrates including those with secondary structure. PNPase also exists in a transient complex with RhlB alone [43]. The helicase activity of RhlB facilitates unwinding of RNA duplexes, allowing PNPase to degrade structured substrates. Importantly, the eukaryotic exosome is also associated with helicases, namely Mtr4 in the nucleus [17,39] and the Ski complex in the cytoplasm [4].
Members of the RNR family of 3’ exos have also been found associated with helicases. In the case of the yeast mitochondrial degradosome, the catalytic RNase II homolog, Dss1, is absolutely dependent on the Suv3 helicase subunit for functionality on any RNA substrate [48]. Indeed, the two subunits of this complex have minimal activity when separated from their partner, and decay mediated by the degradosome is ATP-dependent, reflecting the need for the helicase activity to feed the RNA substrate into the active site of Dss1.
Substrate specificity
The majority of 3’ exos are essentially sequence-independent, but can be targeted to appropriate RNA substrates through sequence- or structure-specific interactions. For example, the structure of the archaeal exosome core consisting of Rrp41 and Rrp42 indicates recognition of only the RNA backbone and not of specific nucleotides [45]. However, the core exosome associates with Csl4 and Rrp4 proteins, both of which have RNA-binding domains and may therefore contribute to substrate selection [46]. Indeed, the affinity of the archaeal exosome core for RNA is significantly increased in the presence of Rrp4 [57].
Many 3’ exos have characterized RNA-binding domains that could contribute to substrate recognition (Figure 2). In addition, other RNA-binding factors can recruit the exosome to RNA substrates through protein-protein interactions.
Figure 2. RNA-binding domains in mammalian 3’ exonucleases.
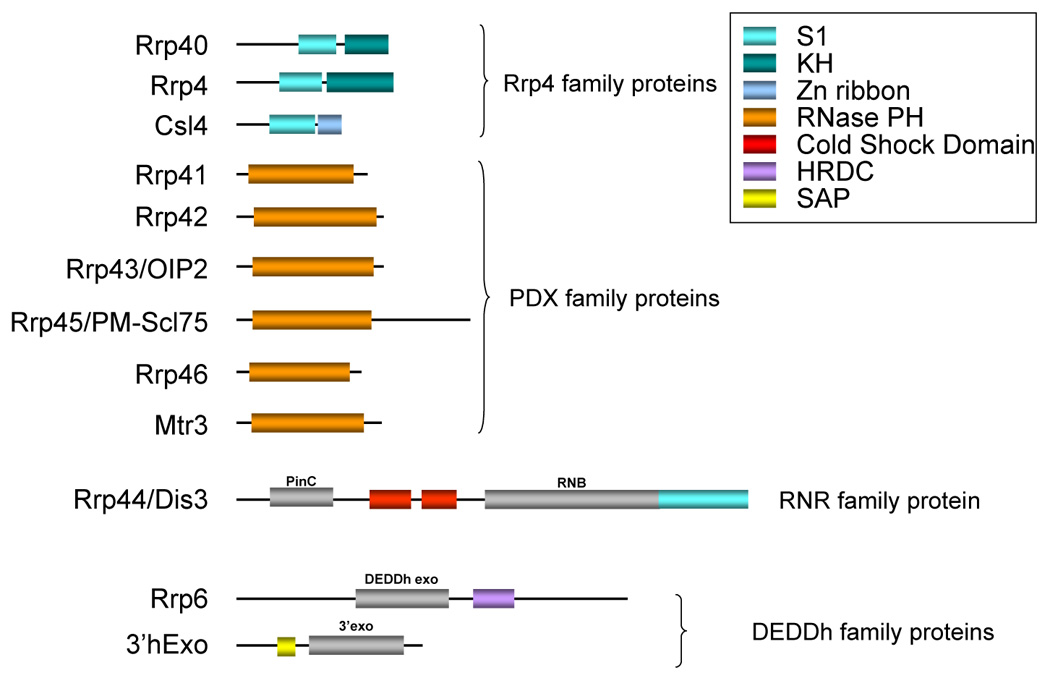
The RNA-binding domains found in various components of the mammalian exosome, as well as 3’hExo are depicted. Exonuclease domains are indicated in grey. The RNAse PH domain is both an RNA-binding domain and an exonuclease domain.
Recently Dis3/Rrp44 was also shown to have the ability to recognize a specific substrate – a hypomodified tRNA that is presumably slightly misfolded [69]. Degradation of this tRNA required both Rrp44 and the nuclear TRAMP complex. However, it remains unclear exactly how Rrp44 recognizes the aberrant tRNA, although amino acids located in the channel leading to the active site appear to be required [69].
Consistent with the instability of AU-rich element containing mRNAs, the PH domain subunits of the exosome, as well as E. coli PNPase, appear to exhibit a binding preference for AU-rich or Urich RNAs [3]. Given that in the reconstituted eukaryotic exosome these subunits are not catalytic it is possible that they serve to recruit the exosome activity to rapidly degrade ARE-containing substrates. The eukaryotic exosome is also able to interact with RNA-binding proteins that recognize AREs, such as the RHAU RNA helicase [78], tristetraprolin [30] and KSRP [26]. This type of interaction serves to recruit the exosome directly to its target RNAs thereby facilitating decay.
The cell may also use the exosome as an antiviral agent. The cellular ZAP protein binds to RNAs of several viruses, including Vesicular Stomatitis Virus and Sindbis Virus, and destabilizes them by recruiting the exosome through an interaction with Rrp46 [29].
The nuclear exosome is recruited specifically to several nuclear RNAs that require processing. Rrp6/PM-Scl100, the catalytic subunit of the exosome in these pathways, has an HRDC domain that has been suggested to be an RNA-binding domain, but the recombinant protein has no RNA-binding activity in vitro [60]. However, Rrp6 may well contribute to substrate preference, as the reconstituted yeast exosome containing Rrp6 exhibits much higher activity on a poly(A) substrate than the complex containing only Rrp44/Dis3 [44]. Recently, two exosome-associated proteins – Rrp47/C1D and MPP6 have been shown to be required for exosome activity in the maturation of 5.8S rRNA [67,68,73]. The yeast Rrp47 protein forms a hexamer with affinity for structured RNA [73] and C1D, the human homolog of Rrp47, has similar RNA binding ability [68]. The human MPP6 protein binds RNA, exhibiting a preference for pyrimidine-rich sequences [67]. Importantly, MPP6 also binds directly to the ITS2 element of pre-rRNAs [67]. Rrp47/C1D associates directly with Rrp6/PM-Scl100 [68,73], while MPP6 interacts with the hMtr4 helicase [68]. Thus, Rrp47/C1D and MPP6 appear to be essential for recognition of specific substrates by the exosome.
In the cytoplasm, the exosome is associated with the Ski complex that may be involved in selection of mRNA substrates for decay. Certainly, one mechanism, the non-stop mRNA decay pathway, requires the Ski complex for substrate recognition [82]. Ski7 is a GTPase present in most, but not all eukaryotes, that resembles the translation factors EF1α and eRF3 [4]. In cases where an mRNA completely lacks a stop codon, the ribosome stalls at the 3’ end of the transcript. Ski7 is thought to insert into the A site of the ribosome and recruit the exosome to initiate decay of these aberrant transcripts. In this case, the exosome accessory factors are recognizing the stalled ribosome rather than a specific sequence or structure in the RNA itself, but the effect is the same.
Processive versus distributive degradation
Structural data has given insight into how 3’ exos attain processivity. In the case of RNase II, the RNA-binding domains form a clamp-like assembly around the RNA body strongly favoring a processive mode of action [94]. Similarly, the high processivity of the archaeal exosome can be explained by the arrangement of three Rrp4 family RNA-binding proteins around a central channel that contains three active sites (Figure 1c) [9,46]. The ring of RNA-binding proteins and central pore secure the RNA, whilst the high concentration of active sites facilitates rapid successive cleavage events possibly even with the RNA molecule rotating between the three active sites. Thus, processivity appears to be achieved by physically trapping the RNA close to the active site. This may explain why many accessory factors, as well as structural components of the exosome have RNA-binding capacity.
Blocking decay
There are two characterized mechanisms to block 3’ exo activity. The first involves modification of the RNA itself such that it is no longer an effective substrate. The second involves association of specific proteins with the RNA. Such proteins could in principle either sterically block 3’ exo access to the RNA, physically interact with the 3’ exo to inhibit its function, or compete for binding with factors that normally recruit a 3’ exo.
RNA modifications abound in both prokaryotic and eukaryotic cells, but their effect on degradation by 3’exos has not been studied in detail. One recent study determined that 2’O-methylation of ribose in rRNAs can inhibit RNase R in Mycoplasma genitalium, although the biological relevance of this observation is unclear [40]. In addition, phosphorothioate modifications have been successfully used to trap 3’-5’ degradation intermediates in vitro consistent with 3’ exos being sensitive to the chemical composition of the RNA [55].
There are several proteins whose association with the substrate RNA appears to prevent 3’ exo action. In eukaryotes, the most obvious example is the cytoplasmic poly(A) binding protein (PABPC1). It might be expected that the ubiquitous 3’ poly(A) tails found on mRNAs would represent excellent 3’ exo substrates in the absence of PABPC1 given their unstructured nature. In eukaryotes, 3’ poly(A) is usually removed by poly(A) specific 3’-5’ exonucleases such as PARN and CCR4, but it is not clear exactly how this process is initiated. Recent data suggest that PABPC1 protein conformation may be altered to induce its dissociation from poly(A) thus allowing the deadenylases to access the substrate [71,90]. Trans-acting factors associated with specific mRNAs may well be able to modulate PABPC1 function to regulate rates of deadenylation.
Following deadenylation, mRNAs are degraded either by decapping and 5’-3’ decay or by the exosome [25]. Interestingly, deadenylation generally leaves a short oligo(A) tail at the 3’ end of the substrate which should present an excellent 3’ single stranded region for recognition by the exosome. However, a heptameric complex of small RNA binding proteins, the Lsm complex, is able to assemble at the 3’ end of deadenylated mRNAs through an affinity for oligo(A) [14]. This protects the 3’ end from 3’ exo activity [76]. Lsm binding favors the alternate 5’-3’ decay pathway and is required for efficient decapping [76]. However, Lsm association may also function to stabilize the transcript in a translationally silent state until such a time as it can be readenylated and returned to polysomes [7]. Recent results from our laboratory indicate that poxviruses may have usurped this function of Lsm proteins to stabilize their own mRNAs against decay [6]. The 5’ poly(A) tract found on many late poxvirus mRNAs associates with Lsm complex and this stabilizes the transcripts against 3’-5’ decay. Interestingly the proteins in the Lsm complex are all closely related to the bacterial Hfq protein which is involved in many aspects of bacterial RNA metabolism, including mRNA decay, and also binds poly(A) [86].
At least two RNA-binding proteins, the Elav protein HuR and the autoantigen La, have been shown to impede 3’ exo activity when bound to RNAs. HuR recognizes AREs, and its association with the TNFα ARE in vitro severely slows 3’-5’ exonucleolytic decay [22]. The mechanism by which inhibition is achieved is unclear, but it may reflect competition with destabilizing factors, such as TTP or KSRP, for the same binding site. La protein associates with the 3’ ends of nascent RNA pol III transcripts, including the pre-tRNAs, and appears to stabilize them against 3’ exo activity, consistent with the absence of La protein on yeast pre-tRNAs undergo 3’ trimming [59,91]. La protein is also necessary for maturation of RNA polymerase II transcripts, namely the U snRNAs and U3 snoRNA [37,49]. As part of their maturation, these transcripts undergo RNase III endonucleolytic cleavage followed by 3’ exonucleolytic trimming to a short U-rich stretch. La association with this U tract prevents excessive trimming through the body of the RNA [36,88].
Interestingly, 3’ poly(U) tracts stabilized RNAs in two independent in vitro 3’ decay assay [23,72]. In this instance, stabilization appeared to be due to trans-acting factors associating with the poly(U) tract and blocking 3’ exo activity as addition of exogenous poly(U) competitor resulted in rapid 3’ exonucleolytic decay [23]. The binding of La protein could not be correlated with stabilization of these transcripts, suggesting that additional factors that bind terminal uridylates could also play a role in stabilizing transcripts from exosome-mediated decay.
Initiating degradation
Endonucleases
The majority of mature RNA molecules in the cell are protected from 3’ exo activity. In order to initiate decay, the protective factor – be it a protein such as PABP, or a secondary structure such as a strong stem-loop – must be neutralized. In prokaryotes, mRNA decay is usually initiated by endonucleolytic cleavage, mediated by RNase E, a component of the degradosome [10]. This functionally inactivates the mRNA, and leads to rapid decay of the 5’ fragment by 3’ exo activity. Endonucleolytic cleavage is a minor mRNA decay pathway in eukaryotes, but is also the initiating step in RNA interference pathways. As mentioned briefly above, 3’ exo activity in the form of the exosome and 3’hExo/ERI-1 is involved in degrading the fragments produced from endonucleolytic cleavage events. Moreover, during processing of many non-coding RNAs, endonucleolytic cleavage events are closely coupled to 3’ exonucleolytic action.
Extending the 3’ end to initiate mRNA decay
As mentioned above, 3’ exos generally require a 3’ terminal single stranded extension for effective substrate recognition. Thus, many substrates need to undergo modification before they can be degraded. In prokaryotes, mRNAs, or their fragments with structured 3’ ends undergo decay through poly(A) addition by poly(A) polymerase, effectively generating the required 3’ extension for initiation of the turnover process. Following polyadenylation the 3’ exos (PNPase, RNAse II, RNase R) can successfully degrade the RNA substrate [10].
A similar process has recently been found to occur in eukaryotic nuclei during degradation of various transcripts including cryptic unstable transcripts, and rRNA and snoRNA precursors [39,87]. TRAMP, a nuclear complex containing a non-canonical poly(A) polymerase, stimulates exosome activity on these structured substrates by providing a 3’ single stranded extension in the form of a poly(A) tail. TRAMP is also likely involved in substrate recognition and has been shown to enable the exosome to distinguish between native and hypomodified tRNAs [69].
Recently, a family of poly(U) polymerases has been identified that are capable of adding oligo(U) extensions to the 3’ end of RNAs [38,64]. These enzymes resemble non-canonical poly(A) polymerases, but their natural substrates are as yet unidentified. It seems likely however that addition of oligo(U) may play a similar role in initiating RNA processing as poly(A). Indeed, cleavage products generated by miRNA action become polyuridylated, and this has been suggested to initiate their degradation [70].
Conclusions and Perspectives
Choosing between processing and degradation
How do 3’exos determine whether a substrate should be trimmed or completely destroyed? It seems likely that trimming is more of a default function achieved by using RNA secondary or tertiary structure to block exonuclease action beyond a certain point. If a substrate is destined for decay, then accessory proteins such as TRAMP or the Ski complex must be recruited to facilitate entry of the structured molecule into the active site of the enzyme. Consistent with this idea, exosome-mediated decay of the body of the RNA substrate has been shown to require ATP in an in vitro assay derived from cytoplasmic extracts [21]. However, it is not at all clear why some select structures or protein complexes might stall 3’ exos. Since all RNAs are typically over 50% double-stranded and usually associated with a number of proteins, it is not clear what constitutes a signal for the 3’ exo to stop degrading. Knowledge of this would be extremely useful for creating mRNAs containing specific blocks to 3’ exo decay which could be used to definitively assess the contribution of this pathway to mRNA turnover in mammalian systems. More work is clearly required to understand exactly how different substrates are recognized by the exosome and the appropriate accessory proteins employed.
How does recruiting the exosome to an RNA result in enhanced decay?
We mentioned several examples above of RNA-binding proteins that associate with the 3’ UTR of unstable transcripts and recruit the exosome, but it is unclear how this event leads to enhanced degradation as factors that normally block exosome activity such as the poly(A) tail or strong secondary structures are presumably still intact. In the case of cytoplasmic mRNA decay, it is possible that exosome recruitment simply serves to divert deadenylated substrates into the 3’ decay pathway, preventing their entry into P bodies for possible readenylation. Therefore the ability for an RNA to interact with the exosome may play a significant role in dictating the fate of the mRNA. Alternatively, the RNA-binding proteins in question may serve as accessory proteins to actively feed the RNA substrate into the active site – this may be the case with a helicase such as RHAU [78].
Novel accessory proteins and elements
Given the large number of RNA metabolism events that 3’ exos are involved in, it seems likely that we have yet to discover all the modes of regulation, or all the factors concerned. Future studies will likely uncover novel sequence and structural elements that influence substrate selection. Global analyses using methodologies such as RIP-CHIP[32] and Ribotrap [5] to elucidate the shared and unique protein components of mRNAs are needed to elucidate both novel factors and regulatory patterns that determine the coordinated fates of mRNA populations. Finally, there are undoubtedly more sequence elements that regulate 3’ exo activity that remain to be discovered. Several overrepresented sequence signatures have been noted in mRNAs that lie near the 3’ end of the transcript [32]. It is possible that these hexamers could represent conserved elements that influence the loading of 3’ exonucleases on RNAs as part of the post-transcriptional regulatory network. It will be interesting to uncover the plethora of ways that RNA substrates have developed to regulate 3’-to-5’ exonuclease activity in cell biology.
Acknowledgements
JW is funded by the National Institutes of Health (GM072481), CJW is funded by the Muscular Dystrophy Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D. Functions of the exosome in rRNA, snoRNA and snRNA synthesis. EMBO J. 1999;18:5399. doi: 10.1093/emboj/18.19.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allmang C, Mitchell P, Petfalski E, Tollervey D. Degradation of ribosomal RNA precursors by the exosome. Nucleic Acids Res. 2000;28:1684. doi: 10.1093/nar/28.8.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JR, Mukherjee D, Muthukumaraswamy K, Moraes KC, Wilusz CJ, Wilusz J. Sequence-specific RNA binding mediated by the RNase PH domain of components of the exosome. RNA. 2006;12:1810. doi: 10.1261/rna.144606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araki Y, Takahashi S, Kobayashi T, Kajiho H, Hoshino S, Katada T. Ski7p G protein interacts with the exosome and the Ski complex for 3′-to-5′ mRNA decay in yeast. EMBO J. 2001;20:4684. doi: 10.1093/emboj/20.17.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach DL, Keene JD. Ribotrap: Targeted purification of RNA-specific RNPs from cell lysates through immunoaffinity precipitation to identify regulatory proteins and RNAs. In: Wilusz J, editor. ‘Post-Transcriptional Gene Regulation’ of Methods in Molecular Biology. Vol. 419. Humana Press; 2008. p. 69. [DOI] [PubMed] [Google Scholar]
- 6.Bergman N, Moraes KC, Anderson JR, Zaric B, Kambach C, Schneider RJ, Wilusz CJ, Wilusz J. Lsm proteins bind and stabilize RNAs containing 5′ poly(A) tracts. Nat. Struct. Mol. Biol. 2007;14:824. doi: 10.1038/nsmb1287. [DOI] [PubMed] [Google Scholar]
- 7.Brengues M, Teixeira D, Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briggs MW, Burkard KT, Butler JS. Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J. Biol. Chem. 1998;273:13255. doi: 10.1074/jbc.273.21.13255. [DOI] [PubMed] [Google Scholar]
- 9.Buttner K, Wenig K, Hopfner KP. Structural framework for the mechanism of archaeal exosomes in RNA processing. Mol. Cell. 2005;20:461. doi: 10.1016/j.molcel.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Carpousis AJ. The RNA Degradosome of Escherichia coli: An mRNA-Degrading Machine Assembled on RNase E. Annu. Rev. Microbiol. 2007;61:71. doi: 10.1146/annurev.micro.61.080706.093440. [DOI] [PubMed] [Google Scholar]
- 11.Chekanova JA, Shaw RJ, Wills MA, Belostotsky DA. Poly(A) tail-dependent exonuclease AtRrp41p from Arabidopsis thaliana rescues 5.8 S rRNA processing and mRNA decay defects of the yeast ski6 mutant and is found in an exosome-sized complex in plant and yeast cells. J. Biol. Chem. 2000;275:33158. doi: 10.1074/jbc.M005493200. [DOI] [PubMed] [Google Scholar]
- 12.Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 13.Cheng ZF, Deutscher MP. An important role for RNase R in mRNA decay. Mol. Cell. 2005;17:313. doi: 10.1016/j.molcel.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury A, Mukhopadhyay J, Tharun S. The decapping activator Lsm1p-7p-Pat1p complex has the intrinsic ability to distinguish between oligoadenylated and polyadenylated RNAs. RNA. 2007;13:998. doi: 10.1261/rna.502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coburn GA, Mackie GA. Overexpression, purification, and properties of Escherichia coli ribonuclease II. J. Biol. Chem. 1996;271:1048. doi: 10.1074/jbc.271.2.1048. [DOI] [PubMed] [Google Scholar]
- 16.Coburn GA, Miao X, Briant DJ, Mackie GA. Reconstitution of a minimal RNA degradosome demonstrates functional coordination between a 3′ exonuclease and a DEAD-box RNA helicase. Genes Dev. 1999;13:2594. doi: 10.1101/gad.13.19.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la CJ, Kressler D, Tollervey D, Linder P. Dob1p (Mtr4p) is a putative ATP-dependent RNA helicase required for the 3′ end formation of 5.8S rRNA in Saccharomyces cerevisiae. EMBO J. 1998;17:1128. doi: 10.1093/emboj/17.4.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominski Z, Yang XC, Kaygun H, Dadlez M, Marzluff WF. A 3′ exonuclease that specifically interacts with the 3′ end of histone mRNA. Mol. Cell. 2003;12:295. doi: 10.1016/s1097-2765(03)00278-8. [DOI] [PubMed] [Google Scholar]
- 19.Duchaine TF, Wohlschlegel JA, Kennedy S, Bei Y, Conte D, Jr, Pang K, Brownell DR, Harding S, Mitani S, Ruvkun G, Yates JR, III, Mello CC. Functional proteomics reveals the biochemical niche of C. elegans DCR-1 in multiple small-RNA-mediated pathways. Cell. 2006;124:343. doi: 10.1016/j.cell.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Dziembowski A, Lorentzen E, Conti E, Seraphin B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol. 2007;14:15. doi: 10.1038/nsmb1184. [DOI] [PubMed] [Google Scholar]
- 21.Espert L, Degols G, Gongora C, Blondel D, Williams BR, Silverman RH, Mechti N. ISG20, a new interferon-induced RNase specific for single-stranded RNA, defines an alternative antiviral pathway against RNA genomic viruses. J. Biol. Chem. 2003;278:16151. doi: 10.1074/jbc.M209628200. [DOI] [PubMed] [Google Scholar]
- 22.Ford LP, Watson J, Keene JD, Wilusz J. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes Dev. 1999;13:188. doi: 10.1101/gad.13.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford LP, Wilusz J. 3′-Terminal RNA structures and poly(U) tracts inhibit initiation by a 3′-->5′ exonuclease in vitro. Nucleic Acids Res. 1999;27:1159. doi: 10.1093/nar/27.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazao C, McVey CE, Amblar M, Barbas A, Vonrhein C, Arraiano CM, Carrondo MA. Unravelling the dynamics of RNA degradation by ribonuclease II and its RNA-bound complex. Nature. 2006;443:110. doi: 10.1038/nature05080. [DOI] [PubMed] [Google Scholar]
- 25.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell Biol. 2007;8:113. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 26.Gherzi R, Lee KY, Briata P, Wegmuller D, Moroni C, Karin M, Chen CY. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol. Cell. 2004;14:571. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh S, Deutscher MP. Oligoribonuclease is an essential component of the mRNA decay pathway. Proc. Natl. Acad. Sci. USA. 1999;96:4372. doi: 10.1073/pnas.96.8.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham AC, Kiss DL, Andrulis ED. Differential distribution of exosome subunits at the nuclear lamina and in cytoplasmic foci. Mol. Biol. Cell. 2006;17:1399. doi: 10.1091/mbc.E05-08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X, Ma J, Sun J, Gao G. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl. Acad. Sci. USA. 2007;104:151. doi: 10.1073/pnas.0607063104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hau HH, Walsh RJ, Ogilvie RL, Williams DA, Reilly CS, Bohjanen PR. Tristetraprolin recruits functional mRNA decay complexes to ARE sequences. J. Cell Biochem. 2007;100:1477. doi: 10.1002/jcb.21130. [DOI] [PubMed] [Google Scholar]
- 31.Houalla R, Devaux F, Fatica A, Kufel J, Barrass D, Torchet C, Tollervey D. Microarray detection of novel nuclear RNA substrates for the exosome. Yeast. 2006;23:439. doi: 10.1002/yea.1369. [DOI] [PubMed] [Google Scholar]
- 32.Hu J, Lutz CS, Wilusz J, Tian B. Bioinformatic identification of candidate cis-regulatory elements involved in human mRNA polyadenylation. RNA. 2005;11:1485. doi: 10.1261/rna.2107305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keene JD, Komisarow JM, Friedersdorf MB. RIP-Chip: the isolation and identification of mRNAs, microRNAs and protein components of ribonucleoprotein complexes from cell extracts. Nat. Protoc. 2006;1:302. doi: 10.1038/nprot.2006.47. [DOI] [PubMed] [Google Scholar]
- 34.Kelly KO, Reuven NB, Li Z, Deutscher MP. RNase PH is essential for tRNA processing and viability in RNase-deficient Escherichia coli cells. J. Biol. Chem. 1992;267:16015. [PubMed] [Google Scholar]
- 35.Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature. 2004;427:645. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- 36.Kufel J, Allmang C, Chanfreau G, Petfalski E, Lafontaine DL, Tollervey D. Precursors to the U3 small nucleolar RNA lack small nucleolar RNP proteins but are stabilized by La binding. Mol. Cell Biol. 2000;20:5415. doi: 10.1128/mcb.20.15.5415-5424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kufel J, Allmang C, Verdone L, Beggs J, Tollervey D. A complex pathway for 3′ processing of the yeast U3 snoRNA. Nucleic Acids Res. 2003;31:6788. doi: 10.1093/nar/gkg904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak JE, Wickens M. A family of poly(U) polymerases. RNA. 2007;13:860. doi: 10.1261/rna.514007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D. RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell. 2005;121:713. doi: 10.1016/j.cell.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Lalonde MS, Zuo Y, Zhang J, Gong X, Wu S, Malhotra A, Li Z. Exoribonuclease R in Mycoplasma genitalium can carry out both RNA processing and degradative functions and is sensitive to RNA ribose methylation. RNA. 2007;13:1957. doi: 10.1261/rna.706207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Z, Deutscher MP. The role of individual exoribonucleases in processing at the 3′ end of Escherichia coli tRNA precursors. J. Biol. Chem. 1994;269:6064. [PubMed] [Google Scholar]
- 42.Li Z, Pandit S, Deutscher MP. 3′ exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1998;95:2856. doi: 10.1073/pnas.95.6.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liou GG, Chang HY, Lin CS, Lin-Chao S. DEAD box RhlB RNA helicase physically associates with exoribonuclease PNPase to degrade double-stranded RNA independent of the degradosome-assembling region of RNase E. J. Biol. Chem. 2002;277:41157. doi: 10.1074/jbc.M206618200. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q, Greimann JC, Lima CD. Reconstitution, activities, and structure of the eukaryotic RNA exosome. Cell. 2006;127:1223. doi: 10.1016/j.cell.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 45.Lorentzen E, Conti E. Structural basis of 3′ end RNA recognition and exoribonucleolytic cleavage by an exosome RNase PH core. Mol. Cell. 2005;20:473. doi: 10.1016/j.molcel.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 46.Lorentzen E, Dziembowski A, Lindner D, Seraphin B, Conti E. RNA channelling by the archaeal exosome. EMBO Rep. 2007;8:470. doi: 10.1038/sj.embor.7400945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lorentzen E, Walter P, Fribourg S, Evguenieva-Hackenberg E, Klug G, Conti E. The archaeal exosome core is a hexameric ring structure with three catalytic subunits. Nat. Struct. Mol. Biol. 2005;12:575. doi: 10.1038/nsmb952. [DOI] [PubMed] [Google Scholar]
- 48.Malecki M, Jedrzejczak R, Stepien PP, Golik P. In vitro reconstitution and characterization of the yeast mitochondrial degradosome complex unravels tight functional interdependence. J. Mol. Biol. 2007;372:23. doi: 10.1016/j.jmb.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 49.Maraia RJ, Intine RV. La protein and its associated small nuclear and nucleolar precursor RNAs. Gene Expr. 2002;10:41. [PMC free article] [PubMed] [Google Scholar]
- 50.Milligan L, Torchet C, Allmang C, Shipman T, Tollervey D. A nuclear surveillance pathway for mRNAs with defective polyadenylation. Mol. Cell Biol. 2005;25:9996. doi: 10.1128/MCB.25.22.9996-10004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohanty BK, Kushner SR. Polynucleotide phosphorylase, RNase II and RNase E play different roles in the in vivo modulation of polyadenylation in Escherichia coli. Mol. Microbiol. 2000;36:982. doi: 10.1046/j.1365-2958.2000.01921.x. [DOI] [PubMed] [Google Scholar]
- 52.Mohanty BK, Kushner SR. Genomic analysis in Escherichia coli demonstrates differential roles for polynucleotide phosphorylase and RNase II in mRNA abundance and decay. Mol. Microbiol. 2003;50:645. doi: 10.1046/j.1365-2958.2003.03724.x. [DOI] [PubMed] [Google Scholar]
- 53.Morl M, Marchfelder A. The final cut. The importance of tRNA 3′-processing. EMBO Rep. 2001;2:17. doi: 10.1093/embo-reports/kve006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moser MJ, Holley WR, Chatterjee A, Mian IS. The proofreading domain of Escherichia coli DNA polymerase I and other DNA and/or RNA exonuclease domains. Nucleic Acids Res. 1997;25:5110. doi: 10.1093/nar/25.24.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mukherjee D, Gao M, O'Connor JP, Raijmakers R, Pruijn G, Lutz CS, Wilusz J. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 2002;21:165. doi: 10.1093/emboj/21.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mullen TE, Marzluff WF. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5’ to 3’ and 3’ to 5’. Genes Dev. 2007 doi: 10.1101/gad.1622708. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oddone A, Lorentzen E, Basquin J, Gasch A, Rybin V, Conti E, Sattler M. Structural and biochemical characterization of the yeast exosome component Rrp40. EMBO Rep. 2007;8:63. doi: 10.1038/sj.embor.7400856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orban TI, Izaurralde E. Decay of mRNAs targeted by RISC requires XRN1, the Ski complex, and the exosome. RNA. 2005;11:459. doi: 10.1261/rna.7231505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pannone BK, Xue D, Wolin SL. A role for the yeast La protein in U6 snRNP assembly: evidence that the La protein is a molecular chaperone for RNA polymerase III transcripts. EMBO J. 1998;17:7442. doi: 10.1093/emboj/17.24.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips S, Butler JS. Contribution of domain structure to the RNA 3′ end processing and degradation functions of the nuclear exosome subunit Rrp6p. RNA. 2003;9:1098. doi: 10.1261/rna.5560903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Proudfoot N. New perspectives on connecting messenger RNA 3′ end formation to transcription. Curr. Opin. Cell Biol. 2004;16:272. doi: 10.1016/j.ceb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 62.Purusharth RI, Madhuri B, Ray MK. Exoribonuclease R in Pseudomonas syringae is essential for growth at low temperature and plays a novel role in the 3′ end processing of 16 and 5 S ribosomal RNA. J. Biol. Chem. 2007;282:16267. doi: 10.1074/jbc.M605588200. [DOI] [PubMed] [Google Scholar]
- 63.Reeder RH, Lang WH. Terminating transcription in eukaryotes: lessons learned from RNA polymerase I. Trends Biochem. Sci. 1997;22:473. doi: 10.1016/s0968-0004(97)01133-x. [DOI] [PubMed] [Google Scholar]
- 64.Rissland OS, Mikulasova A, Norbury CJ. Efficient RNA polyuridylation by noncanonical poly(A) polymerases. Mol. Cell Biol. 2007;27:3612. doi: 10.1128/MCB.02209-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarkar D, Fisher PB. Human polynucleotide phosphorylase (hPNPase old-35): an RNA degradation enzyme with pleiotrophic biological effects. Cell Cycle. 2006;5:2080. doi: 10.4161/cc.5.10.2741. [DOI] [PubMed] [Google Scholar]
- 66.Sarkar D, Leszczyniecka M, Kang DC, Lebedeva IV, Valerie K, Dhar S, Pandita TK, Fisher PB. Down-regulation of Myc as a potential target for growth arrest induced by human polynucleotide phosphorylase (hPNPaseold-35) in human melanoma cells. J. Biol. Chem. 2003;278:24542. doi: 10.1074/jbc.M302421200. [DOI] [PubMed] [Google Scholar]
- 67.Schilders G, Raijmakers R, Raats JM, Pruijn GJ. MPP6 is an exosome-associated RNA-binding protein involved in 5.8S rRNA maturation. Nucleic Acids Res. 2005;33:6795. doi: 10.1093/nar/gki982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schilders G, van DE, Pruijn GJ. C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res. 2007;35:2564. doi: 10.1093/nar/gkm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider C, Anderson JT, Tollervey D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol. Cell. 2007;27:324. doi: 10.1016/j.molcel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shen B, Goodman HM. Uridine addition after microRNA-directed cleavage. Science. 2004;306:997. doi: 10.1126/science.1103521. [DOI] [PubMed] [Google Scholar]
- 71.Simon E, Seraphin B. A specific role for the C-terminal region of the Poly(A)-binding protein in mRNA decay. Nucleic Acids Res. 2007 doi: 10.1093/nar/gkm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song MG, Kiledjian M. 3′ Terminal oligo U-tract-mediated stimulation of decapping. RNA. 2007 doi: 10.1261/rna.765807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stead JA, Costello JL, Livingstone MJ, Mitchell P. The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein. Nucleic Acids Res. 2007;35:5556. doi: 10.1093/nar/gkm614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Symmons MF, Williams MG, Luisi BF, Jones GH, Carpousis AJ. Running rings around RNA: a superfamily of phosphate-dependent RNases. Trends Biochem. Sci. 2002;27:11. doi: 10.1016/s0968-0004(01)01999-5. [DOI] [PubMed] [Google Scholar]
- 75.Takahashi S, Araki Y, Sakuno T, Katada T. Interaction between Ski7p and Upf1p is required for nonsense-mediated 3′-to-5′ mRNA decay in yeast. EMBO J. 2003;22:3951. doi: 10.1093/emboj/cdg374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tharun S, Muhlrad D, Chowdhury A, Parker R. Mutations in the Saccharomyces cerevisiae LSM1 gene that affect mRNA decapping and 3′ end protection. Genetics. 2005;170:33. doi: 10.1534/genetics.104.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Torchet C, Bousquet-Antonelli C, Milligan L, Thompson E, Kufel J, Tollervey D. Processing of 3′-extended read-through transcripts by the exosome can generate functional mRNAs. Mol. Cell. 2002;9:1285. doi: 10.1016/s1097-2765(02)00544-0. [DOI] [PubMed] [Google Scholar]
- 78.Tran H, Schilling M, Wirbelauer C, Hess D, Nagamine Y. Facilitation of mRNA deadenylation and decay by the exosome-bound, DExH protein RHAU. Mol. Cell. 2004;13:101. doi: 10.1016/s1097-2765(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 79.van Hoof A, Frischmeyer PA, Dietz HC, Parker R. Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science. 2002;295:2262. doi: 10.1126/science.1067272. [DOI] [PubMed] [Google Scholar]
- 80.van Hoof A, Lennertz P, Parker R. Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol. Cell Biol. 2000;20:441. doi: 10.1128/mcb.20.2.441-452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Hoof A, Lennertz P, Parker R. Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5.8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J. 2000;19:1357. doi: 10.1093/emboj/19.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vasudevan S, Peltz SW, Wilusz CJ. Non-stop decay--a new mRNA surveillance pathway. Bioessays. 2002;24:785. doi: 10.1002/bies.10153. [DOI] [PubMed] [Google Scholar]
- 83.Vincent HA, Deutscher MP. Substrate recognition and catalysis by the exoribonuclease RNase R. J. Biol. Chem. 2006;281:29769. doi: 10.1074/jbc.M606744200. [DOI] [PubMed] [Google Scholar]
- 84.Wang HW, Wang J, Ding F, Callahan K, Bratkowski MA, Butler JS, Nogales E, Ke A. Architecture of the yeast Rrp44 exosome complex suggests routes of RNA recruitment for 3′ end processing. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16844. doi: 10.1073/pnas.0705526104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, Lewis MS, Johnson AW. Domain interactions within the Ski2/3/8 complex and between the Ski complex and Ski7p. RNA. 2005;11:1291. doi: 10.1261/rna.2060405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilusz CJ, Wilusz J. Eukaryotic Lsm proteins: lessons from bacteria. Nat. Struct. Mol. Biol. 2005;12:1031. doi: 10.1038/nsmb1037. [DOI] [PubMed] [Google Scholar]
- 87.Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, Jacquier A. Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell. 2005;121:725. doi: 10.1016/j.cell.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 88.Xue D, Rubinson DA, Pannone BK, Yoo CJ, Wolin SL. U snRNP assembly in yeast involves the La protein. EMBO J. 2000;19:1650. doi: 10.1093/emboj/19.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang XC, Purdy M, Marzluff WF, Dominski Z. Characterization of 3′hExo, a 3′ exonuclease specifically interacting with the 3′ end of histone mRNA. J. Biol. Chem. 2006;281:30447. doi: 10.1074/jbc.M602947200. [DOI] [PubMed] [Google Scholar]
- 90.Yao G, Chiang YC, Zhang C, Lee DJ, Laue TM, Denis CL. PAB1 self-association precludes its binding to poly(A), thereby accelerating CCR4 deadenylation in vivo. Mol. Cell Biol. 2007;27:6243. doi: 10.1128/MCB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoo CJ, Wolin SL. The yeast La protein is required for the 3′ endonucleolytic cleavage that matures tRNA precursors. Cell. 1997;89:393. doi: 10.1016/s0092-8674(00)80220-2. [DOI] [PubMed] [Google Scholar]
- 92.Zuo Y, Deutscher MP. Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 2001;29:1017. doi: 10.1093/nar/29.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zuo Y, Deutscher MP. The physiological role of RNase T can be explained by its unusual substrate specificity. J. Biol. Chem. 2002;277:29654. doi: 10.1074/jbc.M204252200. [DOI] [PubMed] [Google Scholar]
- 94.Zuo Y, Vincent HA, Zhang J, Wang Y, Deutscher MP, Malhotra A. Structural basis for processivity and single-strand specificity of RNase II. Mol. Cell. 2006;24:149. doi: 10.1016/j.molcel.2006.09.004. [DOI] [PubMed] [Google Scholar]


