Abstract
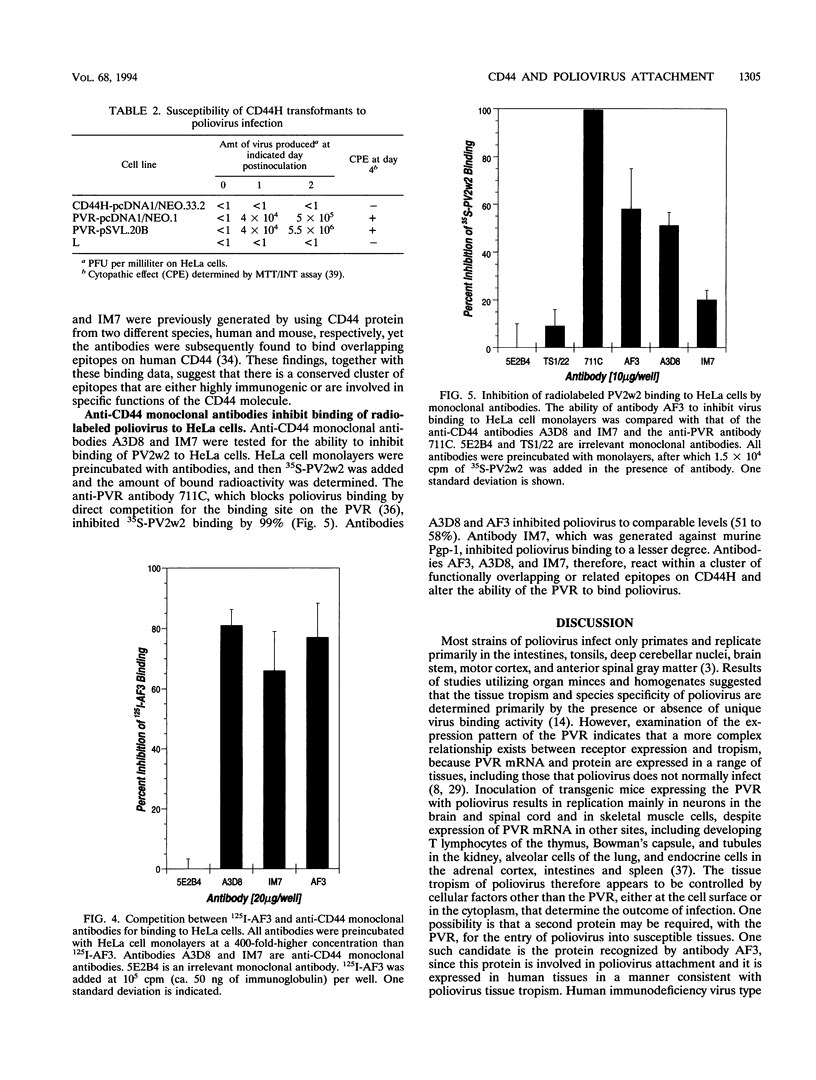
A monoclonal antibody, AF3, was previously shown to specifically inhibit poliovirus binding to HeLa cells and to detect a 100-kDa glycoprotein only in cell lines and tissues permissive for poliovirus infection. These results suggested that the 100-kDa protein may be involved in the pathogenesis of poliomyelitis and the cellular function of the poliovirus receptor site. To study further the role of the 100-kDa protein in poliovirus attachment, immunoaffinity purification, amino acid sequencing, and cDNA cloning were undertaken. The results demonstrate that antibody AF3 reacts with the lymphocyte homing receptor CD44, a multifunctional cell surface glycoprotein involved in the homing of circulating lymphocytes to lymph nodes and the modulation of lymphocyte adhesion and activation. Antibody AF3 reacts with a subset of CD44 molecules (AF3CD44H), which appears to be a small fraction of the heterogeneously glycosylated CD44 molecules expressed on hematopoietic and nonhematopoietic cells. Anti-CD44 monoclonal antibodies, previously reported to induce CD44-mediated modulation of lymphocyte activation and adhesion, compete with 125I-AF3 in binding assays, demonstrating functional overlap among the epitopes. The anti-CD44 monoclonal antibody A3D8, which binds to a greater molecular weight range of CD44 than does AF3, inhibits poliovirus binding to a similar degree. CD44 does not act as a poliovirus receptor, since CD44-expressing mouse L-cell transformants did not bind poliovirus. The poliovirus receptor and AF3CD44H may be noncovalently associated, or they may interact through the cytoskeleton or signal transduction pathways.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Belitsos P. C., Hildreth J. E., August J. T. Homotypic cell aggregation induced by anti-CD44(Pgp-1) monoclonal antibodies and related to CD44(Pgp-1) expression. J Immunol. 1990 Mar 1;144(5):1661–1670. [PubMed] [Google Scholar]
- Carter W. G. The cooperative role of the transformation-sensitive glycoproteins, GP140 and fibronectin, in cell attachment and spreading. J Biol Chem. 1982 Mar 25;257(6):3249–3257. [PubMed] [Google Scholar]
- Dalchau R., Kirkley J., Fabre J. W. Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W 3/13 antigen of the rat. Eur J Immunol. 1980 Oct;10(10):745–749. doi: 10.1002/eji.1830101004. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Le P. T., Singer K. H., Haynes B. F. Antibodies against the CD44 p80, lymphocyte homing receptor molecule augment human peripheral blood T cell activation. J Immunol. 1990 Jan 1;144(1):7–15. [PubMed] [Google Scholar]
- Dougherty G. J., Landorp P. M., Cooper D. L., Humphries R. K. Molecular cloning of CD44R1 and CD44R2, two novel isoforms of the human CD44 lymphocyte "homing" receptor expressed by hemopoietic cells. J Exp Med. 1991 Jul 1;174(1):1–5. doi: 10.1084/jem.174.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freistadt M. S., Kaplan G., Racaniello V. R. Heterogeneous expression of poliovirus receptor-related proteins in human cells and tissues. Mol Cell Biol. 1990 Nov;10(11):5700–5706. doi: 10.1128/mcb.10.11.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Receptor affinities as major determinants of enterovirus tissue tropisms in humans. Virology. 1961 Nov;15:312–326. doi: 10.1016/0042-6822(61)90363-4. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Haynes B. F., Liao H. X., Patton K. L. The transmembrane hyaluronate receptor (CD44): multiple functions, multiple forms. Cancer Cells. 1991 Sep;3(9):347–350. [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- Huet S., Groux H., Caillou B., Valentin H., Prieur A. M., Bernard A. CD44 contributes to T cell activation. J Immunol. 1989 Aug 1;143(3):798–801. [PubMed] [Google Scholar]
- Hughes E. N., Colombatti A., August J. T. Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J Biol Chem. 1983 Jan 25;258(2):1014–1021. [PubMed] [Google Scholar]
- Hughes E. N., Mengod G., August J. T. Murine cell surface glycoproteins. Characterization of a major component of 80,000 daltons as a polymorphic differentiation antigen of mesenchymal cells. J Biol Chem. 1981 Jul 10;256(13):7023–7027. [PubMed] [Google Scholar]
- Idzerda R. L., Carter W. G., Nottenburg C., Wayner E. A., Gallatin W. M., St John T. Isolation and DNA sequence of a cDNA clone encoding a lymphocyte adhesion receptor for high endothelium. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4659–4663. doi: 10.1073/pnas.86.12.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke C. M., Sauvage C. A., Hyman R., Lesley J., Schulte R., Trowbridge I. S. Identification and characterization of the human Pgp-1 glycoprotein. Immunogenetics. 1986;23(5):326–332. doi: 10.1007/BF00398797. [DOI] [PubMed] [Google Scholar]
- Jalkanen S. T., Bargatze R. F., Herron L. R., Butcher E. C. A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986 Oct;16(10):1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Bargatze R. F., de los Toyos J., Butcher E. C. Lymphocyte recognition of high endothelium: antibodies to distinct epitopes of an 85-95-kD glycoprotein antigen differentially inhibit lymphocyte binding to lymph node, mucosal, or synovial endothelial cells. J Cell Biol. 1987 Aug;105(2):983–990. doi: 10.1083/jcb.105.2.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M., Bargatze R., Tammi M., Butcher E. C. Biochemical properties of glycoproteins involved in lymphocyte recognition of high endothelial venules in man. J Immunol. 1988 Sep 1;141(5):1615–1623. [PubMed] [Google Scholar]
- Jalkanen S., Steere A. C., Fox R. I., Butcher E. C. A distinct endothelial cell recognition system that controls lymphocyte traffic into inflamed synovium. Science. 1986 Aug 1;233(4763):556–558. doi: 10.1126/science.3726548. [DOI] [PubMed] [Google Scholar]
- Koopman G., van Kooyk Y., de Graaff M., Meyer C. J., Figdor C. G., Pals S. T. Triggering of the CD44 antigen on T lymphocytes promotes T cell adhesion through the LFA-1 pathway. J Immunol. 1990 Dec 1;145(11):3589–3593. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- McKenzie J. L., Dalchau R., Fabre J. W. Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J Neurochem. 1982 Nov;39(5):1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Minor P. D., Pipkin P. A., Hockley D., Schild G. C., Almond J. W. Monoclonal antibodies which block cellular receptors of poliovirus. Virus Res. 1984;1(3):203–212. doi: 10.1016/0168-1702(84)90039-x. [DOI] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. Bacteriophage lambda vector for transducing a cDNA clone library into mammalian cells. Mol Cell Biol. 1985 May;5(5):1136–1142. doi: 10.1128/mcb.5.5.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Letarte M., Kagnoff M. F., Isacke C. M. Structural heterogeneity of human Pgp-1 and its relationship with p85. Immunogenetics. 1988;27(6):460–464. doi: 10.1007/BF00364434. [DOI] [PubMed] [Google Scholar]
- Picker L. J., De los Toyos J., Telen M. J., Haynes B. F., Butcher E. C. Monoclonal antibodies against the CD44 [In(Lu)-related p80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol. 1989 Mar 15;142(6):2046–2051. [PubMed] [Google Scholar]
- Picker L. J., Nakache M., Butcher E. C. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989 Aug;109(2):927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Racaniello V. R. Human poliovirus receptor gene expression and poliovirus tissue tropism in transgenic mice. J Virol. 1992 Jan;66(1):296–304. doi: 10.1128/jvi.66.1.296-304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepley M. P., Sherry B., Weiner H. L. Monoclonal antibody identification of a 100-kDa membrane protein in HeLa cells and human spinal cord involved in poliovirus attachment. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7743–7747. doi: 10.1073/pnas.85.20.7743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Van Seventer G. A., Siraganian R., Wahl L., Shaw S. Dual role of the CD44 molecule in T cell adhesion and activation. J Immunol. 1989 Oct 15;143(8):2457–2463. [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Aruffo A., Amiot M., Seed B. The hematopoietic and epithelial forms of CD44 are distinct polypeptides with different adhesion potentials for hyaluronate-bearing cells. EMBO J. 1991 Feb;10(2):343–348. doi: 10.1002/j.1460-2075.1991.tb07955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Eisenbarth G. S., Haynes B. F. Human erythrocyte antigens. Regulation of expression of a novel erythrocyte surface antigen by the inhibitor Lutheran In(Lu) gene. J Clin Invest. 1983 Jun;71(6):1878–1886. doi: 10.1172/JCI110943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telen M. J., Palker T. J., Haynes B. F. Human erythrocyte antigens: II. The In(Lu) gene regulates expression of an antigen on an 80-kilodalton protein of human erythrocytes. Blood. 1984 Sep;64(3):599–606. [PubMed] [Google Scholar]
- Telen M. J., Shehata H., Haynes B. F. Human medullary thymocyte p80 antigen and In(Lu)-related p80 antigen reside on the same protein. Hum Immunol. 1986 Nov;17(3):311–324. doi: 10.1016/0198-8859(86)90283-1. [DOI] [PubMed] [Google Scholar]
- Vogel H., Butcher E. C., Picker L. J. H-CAM expression in the human nervous system: evidence for a role in diverse glial interactions. J Neurocytol. 1992 May;21(5):363–373. doi: 10.1007/BF01191704. [DOI] [PubMed] [Google Scholar]