Abstract
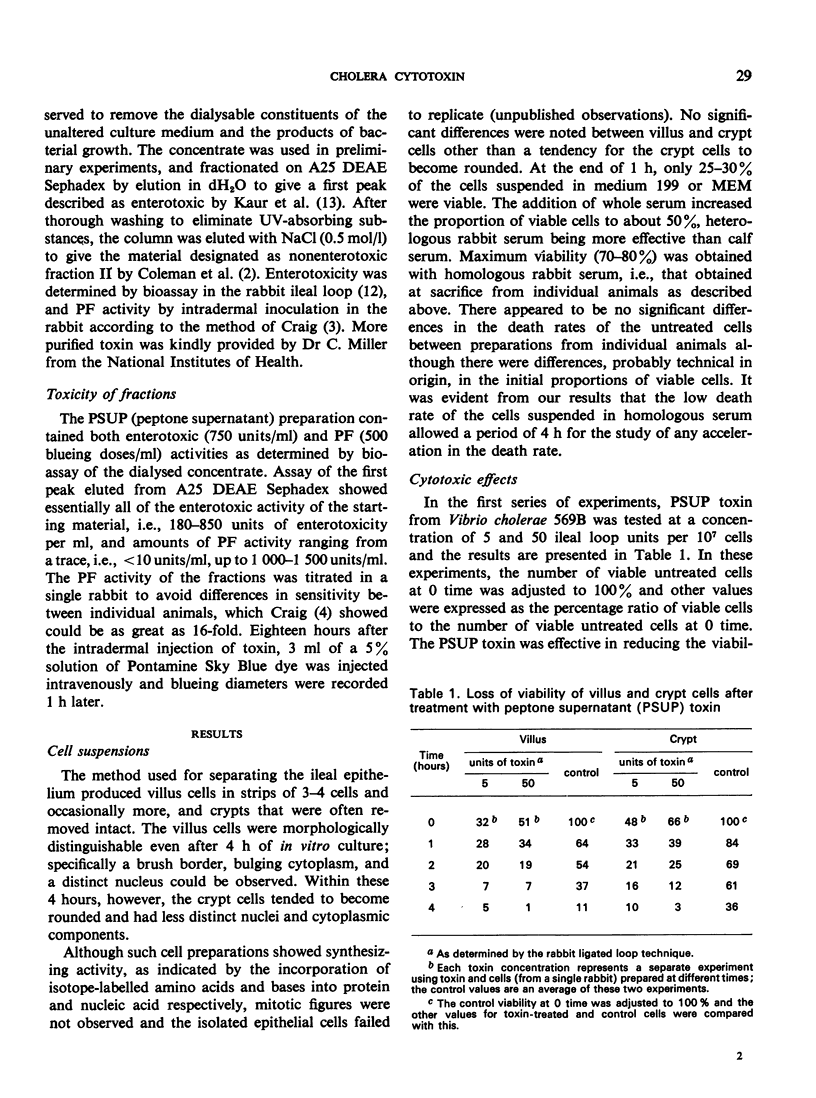
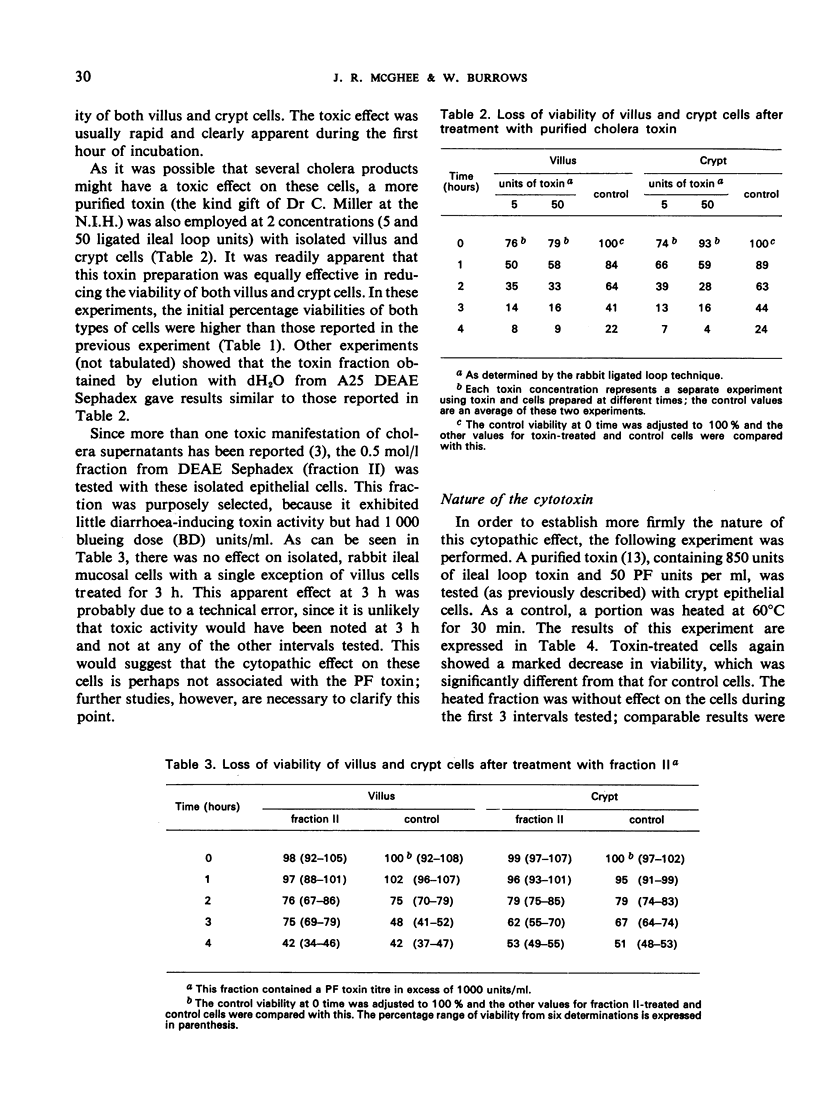
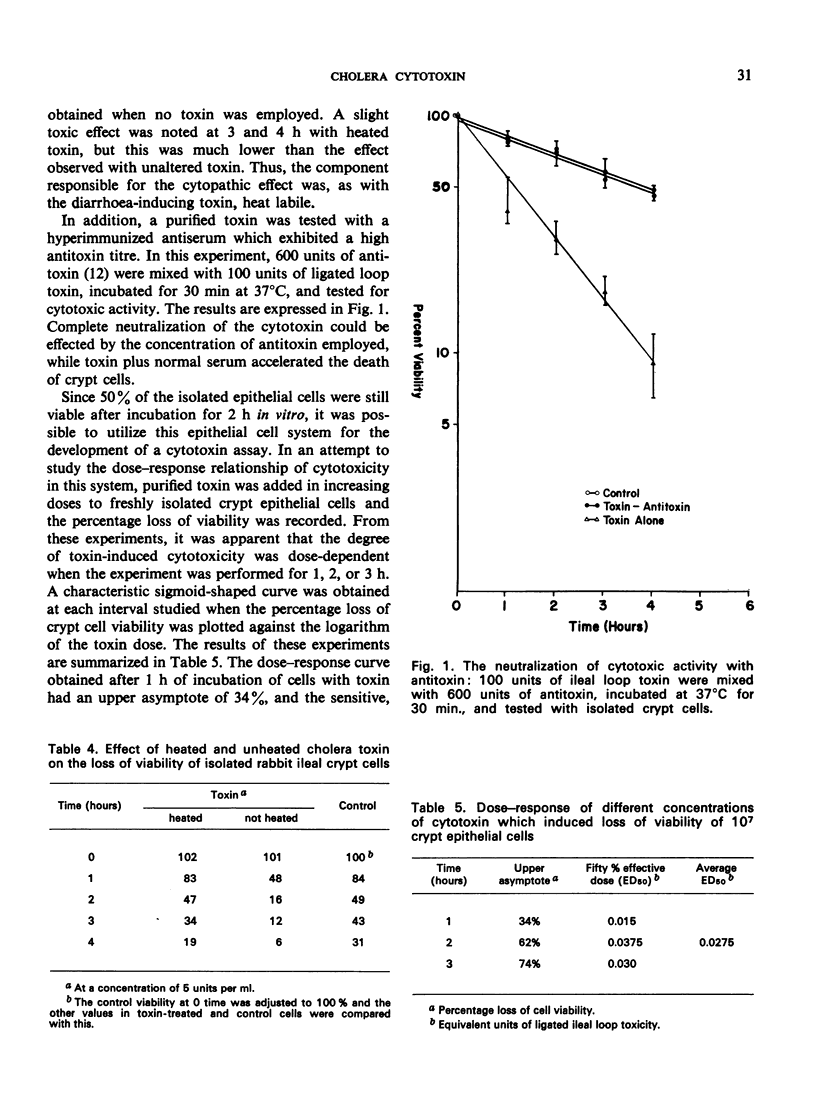
Villus and crypt cells were removed from rabbit ileum by a modification of a method previously described. Villus cells were isolated by vibration of everted bowel segments in citrate buffer, and crypt cells were removed by the expansion of these segments with air and simultaneous vibration in citrate buffer. Several culture media were tested for the maintenance and/or culture of these cells. Two defined media, Eagle's minimum and medium 199, were unsatisfactory and led to rapid cell death. The presence of whole serum was beneficial and both cell types could be maintained for several hours when homologous rabbit serum was employed. Initially 70-80% of villus and crypt cells were viable in homologous serum, and the effect of cholera toxin on cell viability was studied by incubating the cells with peptone dialysate supernatant (PSUP) toxin from Vibrio cholerae for 4 h. PSUP toxin reduced the viability of both villus and crypt cells compared with control preparations, as measured by the uptake of trypan blue; cell death was accelerated with time. A purified, diarrhoea-inducing cholera toxin also reduced the viability of these cells and the results were comparable to those with PSUP toxin. The effect was usually immediate, and a significant reduction in viability occurred within 1 hour of the incubation of cells with toxin. The toxin was heat-labile (60°C/30 min) and nondialysable, and its cytotoxic activity could be completely neutralized with cholera antitoxin. Dose—response studies indicated that as little as 0.0275 equivalent units of ligated ileal loop toxin were active in this system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu Mallick K. C., Bannerjee P. L., Mallik D. C., Ghosh E., Mondol A. Active principles in cholera stool. Indian J Med Res. 1969 Jun;57(6):983–987. [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Donta S. T., King M., Sloper K. Induction of steroidogenesis in tissue culture by cholera enterotoxin. Nat New Biol. 1973 Jun 20;243(129):246–247. doi: 10.1038/newbio243246a0. [DOI] [PubMed] [Google Scholar]
- Elliott H. L., Carpenter C. C., Sack R. B., Yardley J. H. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [PubMed] [Google Scholar]
- HARRER D. S., STERN B. K., REILLY R. W. REMOVAL AND DISSOCIATION OF EPITHELIAL CELLS FROM THE RODENT GASTROINTESTINAL TRACT. Nature. 1964 Jul 18;203:319–320. doi: 10.1038/203319a0. [DOI] [PubMed] [Google Scholar]
- Harrison D. D., Webster H. L. The preparation of isolated intestinal crypt cells. Exp Cell Res. 1969 May;55(2):257–260. doi: 10.1016/0014-4827(69)90489-3. [DOI] [PubMed] [Google Scholar]
- Inwood J., Tyrrell D. A. A cytotoxic factor in cholera toxin. Br J Exp Pathol. 1970 Dec;51(6):597–603. [PMC free article] [PubMed] [Google Scholar]
- Kasai G. J., Burrows W. The titration of cholera toxin and antitoxin in the rabbit ileal loop. J Infect Dis. 1966 Dec;116(5):606–614. doi: 10.1093/infdis/116.5.606. [DOI] [PubMed] [Google Scholar]
- Kaur J., McGhee, Burrows W. Immunity to cholera: the occurrence and nature of antibody-active immunoglobulins in the lower ileum of the rabbit. J Immunol. 1972 Feb;108(2):387–395. [PubMed] [Google Scholar]
- Sultzer B. M., Craig J. P. Cholera toxin inhibits macromolecular synthesis in mouse spleen cells. Nat New Biol. 1973 Aug 8;244(136):178–180. doi: 10.1038/newbio244178a0. [DOI] [PubMed] [Google Scholar]
- TENNANT J. R. EVALUATION OF THE TRYPAN BLUE TECHNIQUE FOR DETERMINATION OF CELL VIABILITY. Transplantation. 1964 Nov;2:685–694. doi: 10.1097/00007890-196411000-00001. [DOI] [PubMed] [Google Scholar]
- Webster H. L., Harrison D. D. Enzymic activities during the transformation of crypt to columnar intestinal cells. Exp Cell Res. 1969 Aug;56(2):245–253. doi: 10.1016/0014-4827(69)90009-3. [DOI] [PubMed] [Google Scholar]


