Abstract
Analyses of different cowpox virus (Brighton Red strain [CPV-BR]) mutants indicate that there is a minimum of three genes encoded by CPV-BR that are nonessential for virus replication in tissue culture but are involved in inhibiting the generation of an inflammatory response in the chicken embryo chorioallantoic membrane (CAM) model. The CPV-BR-encoded anti-inflammatory genes include the gene encoding the 38-kDa protein (also called 38K, crmA, SPI-2, or VV-WR-ORF-B13R), a tumor necrosis factor receptor homolog, and an unidentified gene that maps to the right end of the CPV genome. The kinetics of triggering of an inflammatory response at the site of virus infection as well as the magnitude of the response is dependent on the virus-encoded inhibitor that is deleted. Virus yields recovered from pocks decreased in proportion to the magnitude of the inflammatory response. The deletion of these identified inhibitors of inflammation was associated with attenuation of the mutant viruses in mice. These data confirm the existence of multiple poxvirus-encoded host defense modifiers whose function is to block the generation of an inflammatory response at the site of virus infection, which allows enhanced virus replication and potentially facilitates virus transmission.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson J. S., Giebink G. S., Mills E. L., Quie P. G. Polymorphonuclear leukocyte dysfunction during influenza virus infection in chinchillas. J Infect Dis. 1981 Jun;143(6):836–845. doi: 10.1093/infdis/143.6.836. [DOI] [PubMed] [Google Scholar]
- Alcamí A., Smith G. L. A soluble receptor for interleukin-1 beta encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992 Oct 2;71(1):153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Viruses launch their own 'star wars'. Science. 1992 Dec 11;258(5089):1730–1731. doi: 10.1126/science.1334571. [DOI] [PubMed] [Google Scholar]
- Beattie E., Tartaglia J., Paoletti E. Vaccinia virus-encoded eIF-2 alpha homolog abrogates the antiviral effect of interferon. Virology. 1991 Jul;183(1):419–422. doi: 10.1016/0042-6822(91)90158-8. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Cooper J. A., Twardzik D. R., Moss B. Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol. 1988 Mar;62(3):866–874. doi: 10.1128/jvi.62.3.866-874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Palumbo G. J. Poxvirus pathogenesis. Microbiol Rev. 1991 Mar;55(1):80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Smith G. L., Cremer K., Notkins A. L., Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. 1985 Oct 31-Nov 6Nature. 317(6040):813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Chang H. W., Watson J. C., Jacobs B. L. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4825–4829. doi: 10.1073/pnas.89.11.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Ager L. L., Gartland G. L., Cooper M. D. Identification of a T3/T cell receptor complex in chickens. J Exp Med. 1986 Jul 1;164(1):375–380. doi: 10.1084/jem.164.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Cihak J., Lösch U., Cooper M. D. Differential expression of two T cell receptors, TcR1 and TcR2, on chicken lymphocytes. Eur J Immunol. 1988 Apr;18(4):539–543. doi: 10.1002/eji.1830180408. [DOI] [PubMed] [Google Scholar]
- Chen W., Drillien R., Spehner D., Buller R. M. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992 Apr;187(2):433–442. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- Child S. J., Palumbo G. J., Buller R. M., Hruby D. E. Insertional inactivation of the large subunit of ribonucleotide reductase encoded by vaccinia virus is associated with reduced virulence in vivo. Virology. 1990 Feb;174(2):625–629. doi: 10.1016/0042-6822(90)90119-c. [DOI] [PubMed] [Google Scholar]
- Chua T. P., Smith C. E., Reith R. W., Williamson J. D. Inflammatory responses and the generation of chemoattractant activity in cowpox virus-infected tissues. Immunology. 1990 Feb;69(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Davies M. V., Elroy-Stein O., Jagus R., Moss B., Kaufman R. J. The vaccinia virus K3L gene product potentiates translation by inhibiting double-stranded-RNA-activated protein kinase and phosphorylation of the alpha subunit of eukaryotic initiation factor 2. J Virol. 1992 Apr;66(4):1943–1950. doi: 10.1128/jvi.66.4.1943-1950.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Knight J. C. Orthopoxvirus DNA: a comparison of restriction profiles and maps. Virology. 1985 May;143(1):230–251. doi: 10.1016/0042-6822(85)90111-4. [DOI] [PubMed] [Google Scholar]
- FENNER F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology. 1958 Jun;5(3):502–529. doi: 10.1016/0042-6822(58)90042-4. [DOI] [PubMed] [Google Scholar]
- Fredrickson T. N., Sechler J. M., Palumbo G. J., Albert J., Khairallah L. H., Buller R. M. Acute inflammatory response to cowpox virus infection of the chorioallantoic membrane of the chick embryo. Virology. 1992 Apr;187(2):693–704. doi: 10.1016/0042-6822(92)90472-2. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Virus proteins that counteract host immune defenses. Cell. 1992 Oct 2;71(1):5–7. doi: 10.1016/0092-8674(92)90259-f. [DOI] [PubMed] [Google Scholar]
- Howard S. T., Chan Y. S., Smith G. L. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991 Feb;180(2):633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Isaacs S. N., Kotwal G. J., Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMPE C. H. Studies smallpox and complications of smallpox vaccination. Pediatrics. 1960 Aug;26:176–189. [PubMed] [Google Scholar]
- Karupiah G., Fredrickson T. N., Holmes K. L., Khairallah L. H., Buller R. M. Importance of interferons in recovery from mousepox. J Virol. 1993 Jul;67(7):4214–4226. doi: 10.1128/jvi.67.7.4214-4226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish M. R., King N. J., Woodhams C. E., Ramshaw I. A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990 Jan;20(1):157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Kotwal G. J., Isaacs S. N., McKenzie R., Frank M. M., Moss B. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science. 1990 Nov 9;250(4982):827–830. doi: 10.1126/science.2237434. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Blades R. Impairment of human polymorphonuclear leucocyte function by influenza virus. Lancet. 1976 Feb 7;1(7954):283–283. doi: 10.1016/s0140-6736(76)91407-0. [DOI] [PubMed] [Google Scholar]
- Meyer H., Sutter G., Mayr A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol. 1991 May;72(Pt 5):1031–1038. doi: 10.1099/0022-1317-72-5-1031. [DOI] [PubMed] [Google Scholar]
- O'CONNELL C. J., KARZON D. T., BARRON A. L., PLAUT M. E., ALI V. M. PROGRESSIVE VACCINIA WITH NORMAL ANTIBODIES. A CASE POSSIBLY DUE TO DEFICIENT CELLULAR IMMUNITY. Ann Intern Med. 1964 Feb;60:282–289. doi: 10.7326/0003-4819-60-2-282. [DOI] [PubMed] [Google Scholar]
- Palumbo G. J., Buller R. M. Inhibitors of the lipoxygenase pathway specifically block orthopoxvirus replication. Virology. 1991 Jan;180(1):457–463. doi: 10.1016/0042-6822(91)90058-j. [DOI] [PubMed] [Google Scholar]
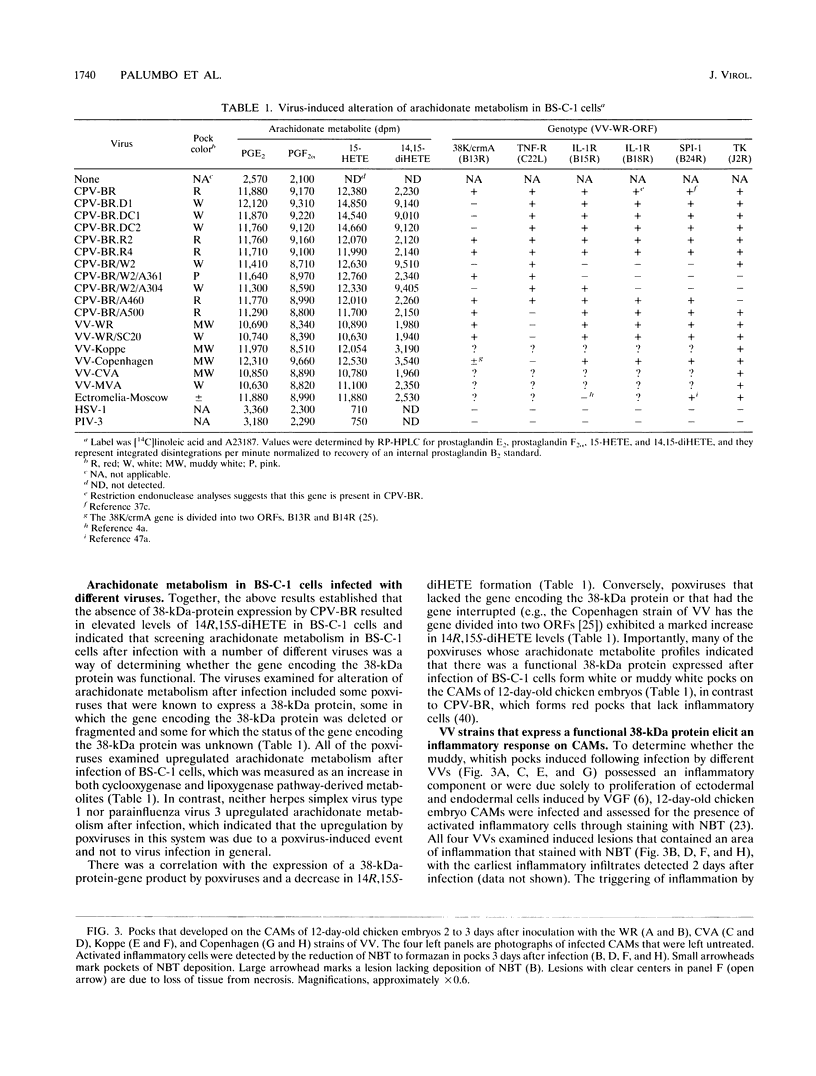
- Palumbo G. J., Glasgow W. C., Buller R. M. Poxvirus-induced alteration of arachidonate metabolism. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2020–2024. doi: 10.1073/pnas.90.5.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo G. J., Pickup D. J., Fredrickson T. N., McIntyre L. J., Buller R. M. Inhibition of an inflammatory response is mediated by a 38-kDa protein of cowpox virus. Virology. 1989 Sep;172(1):262–273. doi: 10.1016/0042-6822(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Pickup D. J., Ink B. S., Hu W., Ray C. A., Joklik W. K. Hemorrhage in lesions caused by cowpox virus is induced by a viral protein that is related to plasma protein inhibitors of serine proteases. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7698–7702. doi: 10.1073/pnas.83.20.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 beta converting enzyme. Cell. 1992 May 15;69(4):597–604. doi: 10.1016/0092-8674(92)90223-y. [DOI] [PubMed] [Google Scholar]
- Ruutu P. Depression of rat neutrophil exudation and motility by influenza virus. Scand J Immunol. 1977;6(11):1113–1120. doi: 10.1111/j.1365-3083.1977.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Sawyer W. D. Interaction of influenza virus with leukocytes and its effect on phagocytosis. J Infect Dis. 1969 Jun;119(6):541–556. doi: 10.1093/infdis/119.6.541. [DOI] [PubMed] [Google Scholar]
- Schlesinger J. J., Ernst C., Weinstein L. Letter: Inhibition of human neutrophil chemotaxis by influenza virus. Lancet. 1976 Mar 20;1(7960):650–651. doi: 10.1016/s0140-6736(76)90471-2. [DOI] [PubMed] [Google Scholar]
- Schröder J. M. The monocyte-derived neutrophil activating peptide (NAP/interleukin 8) stimulates human neutrophil arachidonate-5-lipoxygenase, but not the release of cellular arachidonate. J Exp Med. 1989 Sep 1;170(3):847–863. doi: 10.1084/jem.170.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shak S., Perez H. D., Goldstein I. M. A novel dioxygenation product of arachidonic acid possesses potent chemotactic activity for human polymorphonuclear leukocytes. J Biol Chem. 1983 Dec 25;258(24):14948–14953. [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Chan Y. S. Two vaccinia virus proteins structurally related to the interleukin-1 receptor and the immunoglobulin superfamily. J Gen Virol. 1991 Mar;72(Pt 3):511–518. doi: 10.1099/0022-1317-72-3-511. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Hruby D. E., Maliszewski C. R., Pickup D. J., Sims J. E., Buller R. M., VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992 Oct 2;71(1):145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Upton C., Macen J. L., Schreiber M., McFadden G. Myxoma virus expresses a secreted protein with homology to the tumor necrosis factor receptor gene family that contributes to viral virulence. Virology. 1991 Sep;184(1):370–382. doi: 10.1016/0042-6822(91)90853-4. [DOI] [PubMed] [Google Scholar]
- Upton C., Mossman K., McFadden G. Encoding of a homolog of the IFN-gamma receptor by myxoma virus. Science. 1992 Nov 20;258(5086):1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- VAN TONGEREN H. A. E. Spontaneous mutation of cowpox-virus by means of egg-passage. Arch Gesamte Virusforsch. 1952;5(1):35–52. doi: 10.1007/BF01245138. [DOI] [PubMed] [Google Scholar]