Abstract
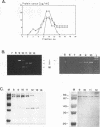
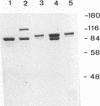
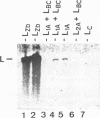
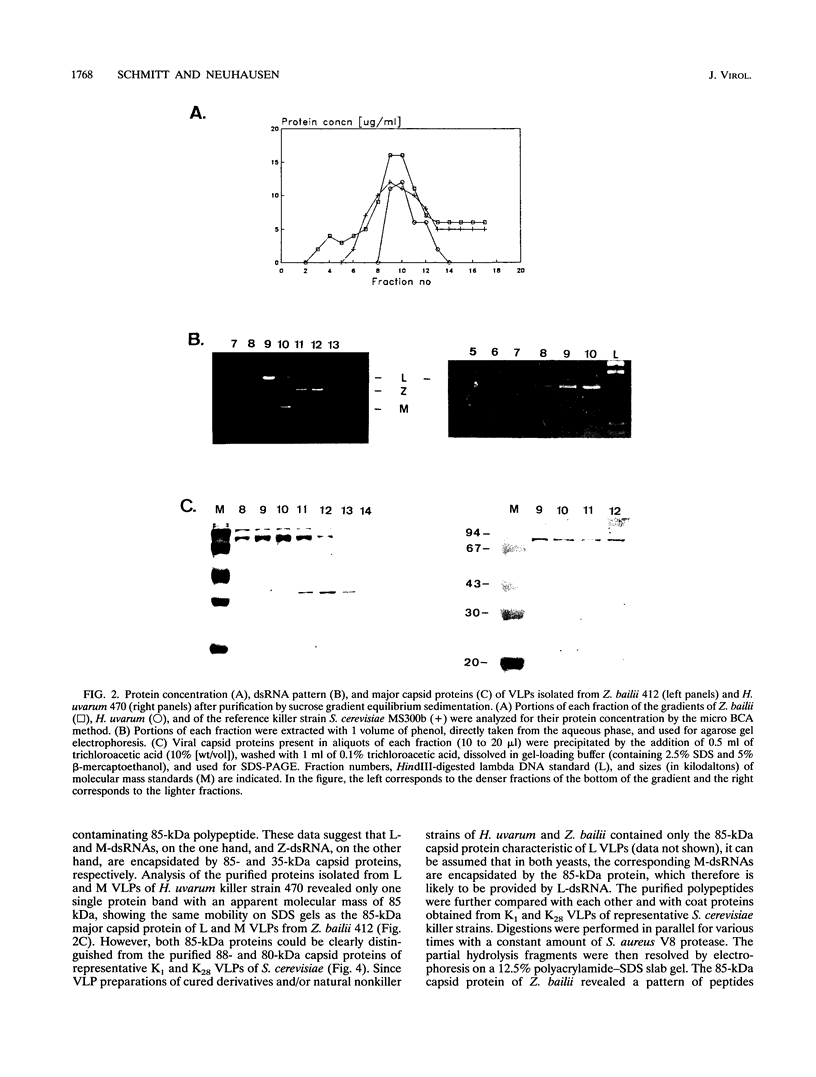
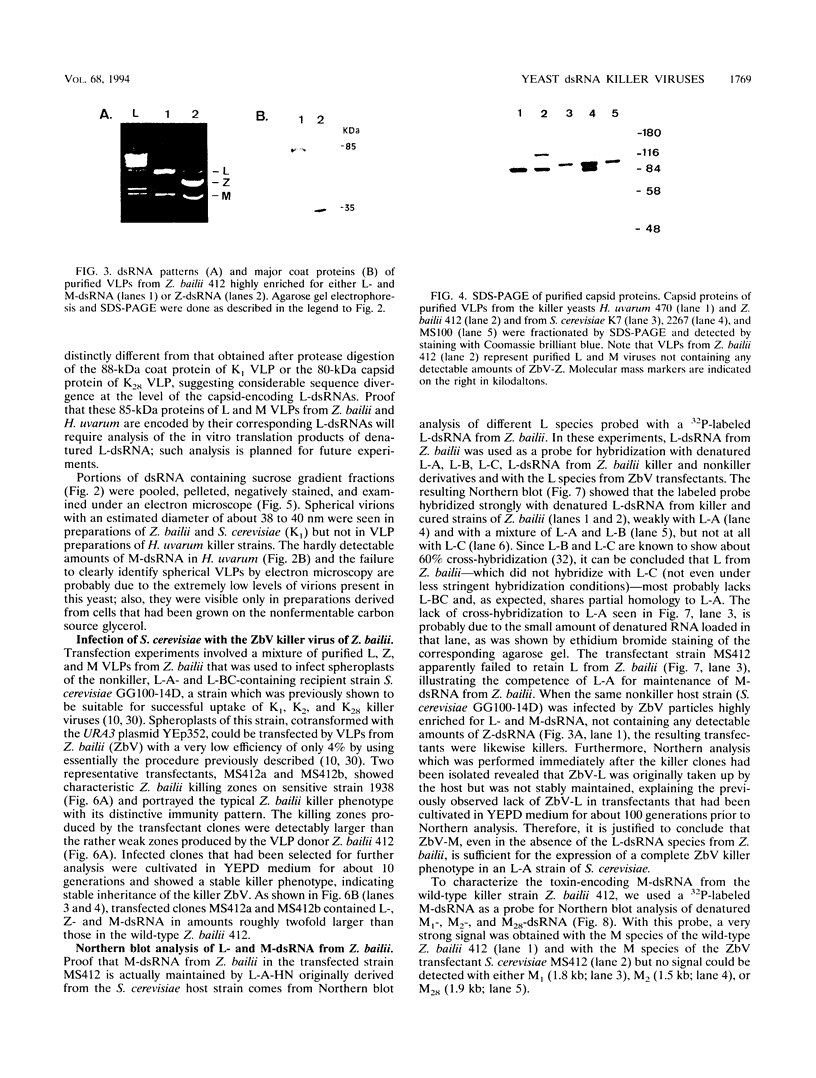
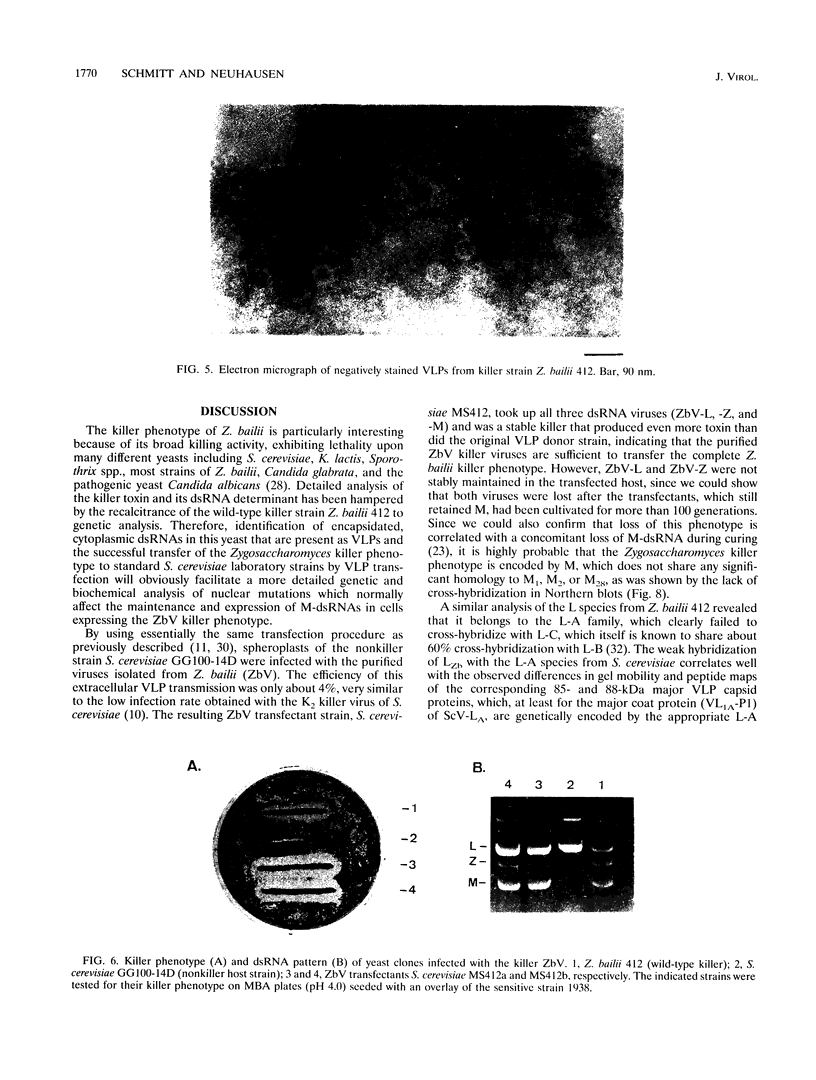
Killer toxin-secreting strains of the yeasts Hanseniaspora uvarum and Zygosaccharomyces bailii were shown to contain linear double-stranded RNAs (dsRNAs) that persist within the cytoplasm of the infected host cell as encapsidated virus-like particles. In both yeasts, L- and M-dsRNAs were associated with 85-kDa major capsid protein, whereas the additional Z-dsRNA (2.8 kb), present only in the wild-type Z. bailii killer strain, was capsid protein, whereas the additional Z-dsRNA (2.8 kb), present only in the wild-type Z. bailii killer strain, was shown to be encapsidated by a 35-kDa coat protein. Although Northern (RNA) blot hybridizations indicated that L-dsRNA from Z. bailii is a LA species, additional peptide maps of the purified 85-kDa capsid from Z. bailii and the 88- and 80-kDa major coat proteins from K1 and K28 killer viruses of Saccharomyces cerevisiae revealed distinctly different patterns of peptides. Electron microscopy of purified Z. bailii viruses (ZbV) identified icosahedral particles 40 nm in diameter which were undistinguishable from the S. cerevisiae killer viruses. We demonstrated that purified ZbVs are sufficient to confer the Z. bailii killer phenotype on transfected spheroplasts of a S. cerevisiae nonkiller strain and that the resulting transfectants secreted even more killer toxin that the original ZbV donor strain did. Curing experiments with ZbV-transfected S. cerevisiae strains indicated that the M-dsRNA satellite from Z. bailii contains the genetic information for toxin production, whereas expression of toxin immunity might be dependent on Z-dsRNA, which resembles a new dsRNA replicon in yeasts that is not dependent on an LA helper virus to be stably maintained and replicated within the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball S. G., Tirtiaux C., Wickner R. B. Genetic Control of L-a and L-(Bc) Dsrna Copy Number in Killer Systems of SACCHAROMYCES CEREVISIAE. Genetics. 1984 Jun;107(2):199–217. doi: 10.1093/genetics/107.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C., Bussey H., Greene D., Thomas D. Y., Vernet T. Yeast killer toxin: site-directed mutations implicate the precursor protein as the immunity component. Cell. 1986 Jul 4;46(1):105–113. doi: 10.1016/0092-8674(86)90864-0. [DOI] [PubMed] [Google Scholar]
- Bussey H. K1 killer toxin, a pore-forming protein from yeast. Mol Microbiol. 1991 Oct;5(10):2339–2343. doi: 10.1111/j.1365-2958.1991.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Bussey H., Sacks W., Galley D., Saville D. Yeast killer plasmid mutations affecting toxin secretion and activity and toxin immunity function. Mol Cell Biol. 1982 Apr;2(4):346–354. doi: 10.1128/mcb.2.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Diamond M. E., Dowhanick J. J., Nemeroff M. E., Pietras D. F., Tu C. L., Bruenn J. A. Overlapping genes in a yeast double-stranded RNA virus. J Virol. 1989 Sep;63(9):3983–3990. doi: 10.1128/jvi.63.9.3983-3990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. Three different M1 RNA-containing viruslike particle types in Saccharomyces cerevisiae: in vitro M1 double-stranded RNA synthesis. Mol Cell Biol. 1986 May;6(5):1552–1561. doi: 10.1128/mcb.6.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Esteban L. M., Wickner R. B. Portable encapsidation signal of the L-A double-stranded RNA virus of S. cerevisiae. Cell. 1990 Aug 24;62(4):819–828. doi: 10.1016/0092-8674(90)90125-x. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Ribas J. C., Makhov A. M., Wickner R. B. Pol of gag-pol fusion protein required for encapsidation of viral RNA of yeast L-A virus. Nature. 1992 Oct 22;359(6397):746–749. doi: 10.1038/359746a0. [DOI] [PubMed] [Google Scholar]
- Gunge N., Sakaguchi K. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J Bacteriol. 1981 Jul;147(1):155–160. doi: 10.1128/jb.147.1.155-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981 Jan;145(1):382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J Biol Chem. 1989 Apr 25;264(12):6716–6723. [PubMed] [Google Scholar]
- Radler F., Herzberger S., Schönig I., Schwarz P. Investigation of a killer strain of Zygosaccharomyces bailii. J Gen Microbiol. 1993 Mar;139(3):495–500. doi: 10.1099/00221287-139-3-495. [DOI] [PubMed] [Google Scholar]
- Radler F., Schmitt M. J., Meyer B. Killer toxin of Hanseniaspora uvarum. Arch Microbiol. 1990;154(2):175–178. doi: 10.1007/BF00423329. [DOI] [PubMed] [Google Scholar]
- Sawant A. D., Abdelal A. T., Ahearn D. G. Purification and characterization of the anti-Candida toxin of Pichia anomala WC 65. Antimicrob Agents Chemother. 1989 Jan;33(1):48–52. doi: 10.1128/aac.33.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. J., Tipper D. J. Genetic analysis of maintenance and expression of L and M double-stranded RNAs from yeast killer virus K28. Yeast. 1992 May;8(5):373–384. doi: 10.1002/yea.320080505. [DOI] [PubMed] [Google Scholar]
- Schmitt M. J., Tipper D. J. K28, a unique double-stranded RNA killer virus of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Sep;10(9):4807–4815. doi: 10.1128/mcb.10.9.4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M., Radler F. Mannoprotein of the yeast cell wall as primary receptor for the killer toxin of Saccharomyces cerevisiae strain 28. J Gen Microbiol. 1987 Dec;133(12):3347–3354. doi: 10.1099/00221287-133-12-3347. [DOI] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Yeast L dsRNA consists of at least three distinct RNAs; evidence that the non-Mendelian genes [HOK], [NEX] and [EXL] are on one of these dsRNAs. Cell. 1982 Dec;31(2 Pt 1):429–441. doi: 10.1016/0092-8674(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Sugisaki Y., Gunge N., Sakaguchi K., Yamasaki M., Tamura G. Transfer of DNA killer plasmids from Kluyveromyces lactis to Kluyveromyces fragilis and Candida pseudotropicalis. J Bacteriol. 1985 Dec;164(3):1373–1375. doi: 10.1128/jb.164.3.1373-1375.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Ginsberg I., Banerjee N., Held W., Koltin Y., Bruenn J. A. Ustilago maydis KP6 killer toxin: structure, expression in Saccharomyces cerevisiae, and relationship to other cellular toxins. Mol Cell Biol. 1990 Apr;10(4):1373–1381. doi: 10.1128/mcb.10.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. J., Leibowitz M. J. Structural and functional analysis of separated strands of killer double-stranded RNA of yeast. Nucleic Acids Res. 1982 Nov 11;10(21):6903–6918. doi: 10.1093/nar/10.21.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Bostian K. A. Double-stranded ribonucleic acid killer systems in yeasts. Microbiol Rev. 1984 Jun;48(2):125–156. doi: 10.1128/mr.48.2.125-156.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Schmitt M. J. Yeast dsRNA viruses: replication and killer phenotypes. Mol Microbiol. 1991 Oct;5(10):2331–2338. doi: 10.1111/j.1365-2958.1991.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Tu C., Tzeng T. H., Bruenn J. A. Ribosomal movement impeded at a pseudoknot required for frameshifting. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8636–8640. doi: 10.1073/pnas.89.18.8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng T. H., Tu C. L., Bruenn J. A. Ribosomal frameshifting requires a pseudoknot in the Saccharomyces cerevisiae double-stranded RNA virus. J Virol. 1992 Feb;66(2):999–1006. doi: 10.1128/jvi.66.2.999-1006.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Meinkoth J. L., Kimmel A. R. Northern and Southern blots. Methods Enzymol. 1987;152:572–581. doi: 10.1016/0076-6879(87)52064-x. [DOI] [PubMed] [Google Scholar]
- Weinstein L. A., Capaldo-Kimball F., Leibowitz M. J. Genetics of heat-curability of killer virus of yeast. Yeast. 1993 Apr;9(4):411–418. doi: 10.1002/yea.320090411. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. "Killer character" of Saccharomyces cerevisiae: curing by growth at elevated temperature. J Bacteriol. 1974 Mar;117(3):1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu Rev Microbiol. 1992;46:347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]
- el-Sherbeini M., Bostian K. A., Levitre J., Mitchell D. J. Gene-protein assignments within the yeast Yarrowia lipolytica dsRNA viral genome. Curr Genet. 1987;11(6-7):483–490. doi: 10.1007/BF00384610. [DOI] [PubMed] [Google Scholar]
- el-Sherbeini M., Bostian K. A. Viruses in fungi: infection of yeast with the K1 and K2 killer viruses. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4293–4297. doi: 10.1073/pnas.84.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]