Abstract
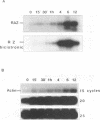
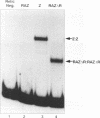
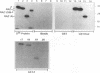
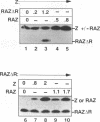
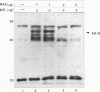
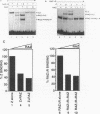
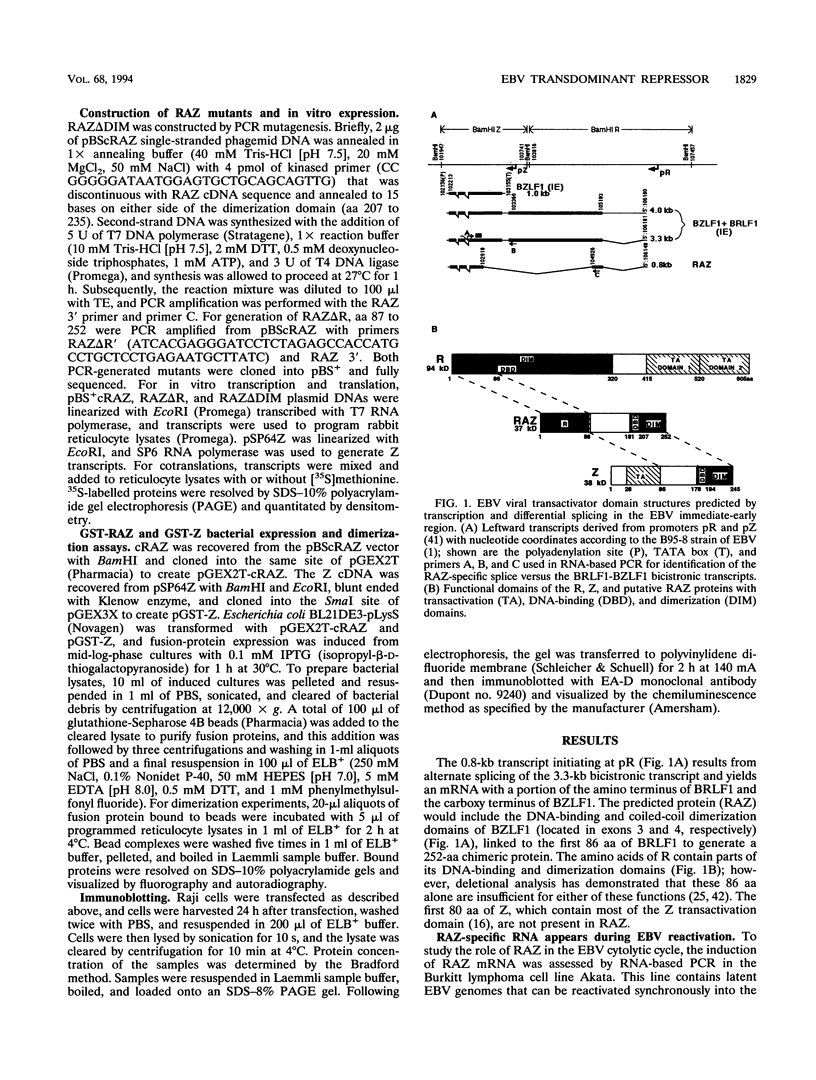
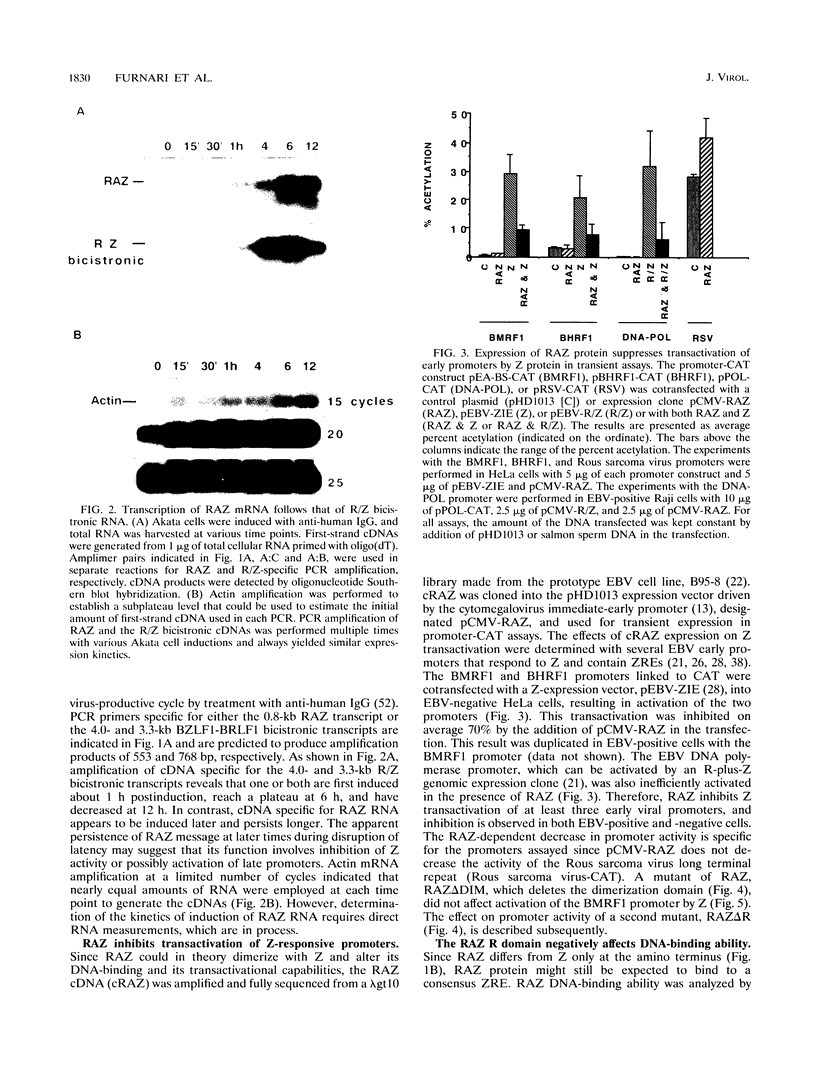
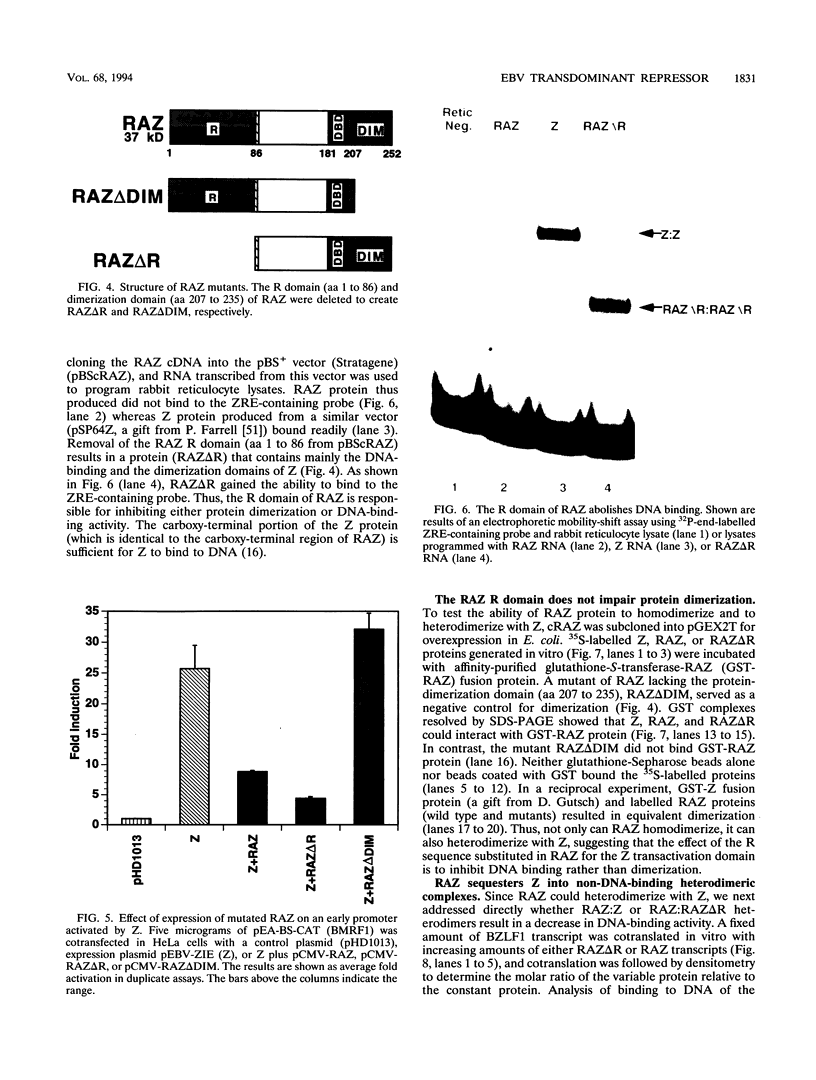
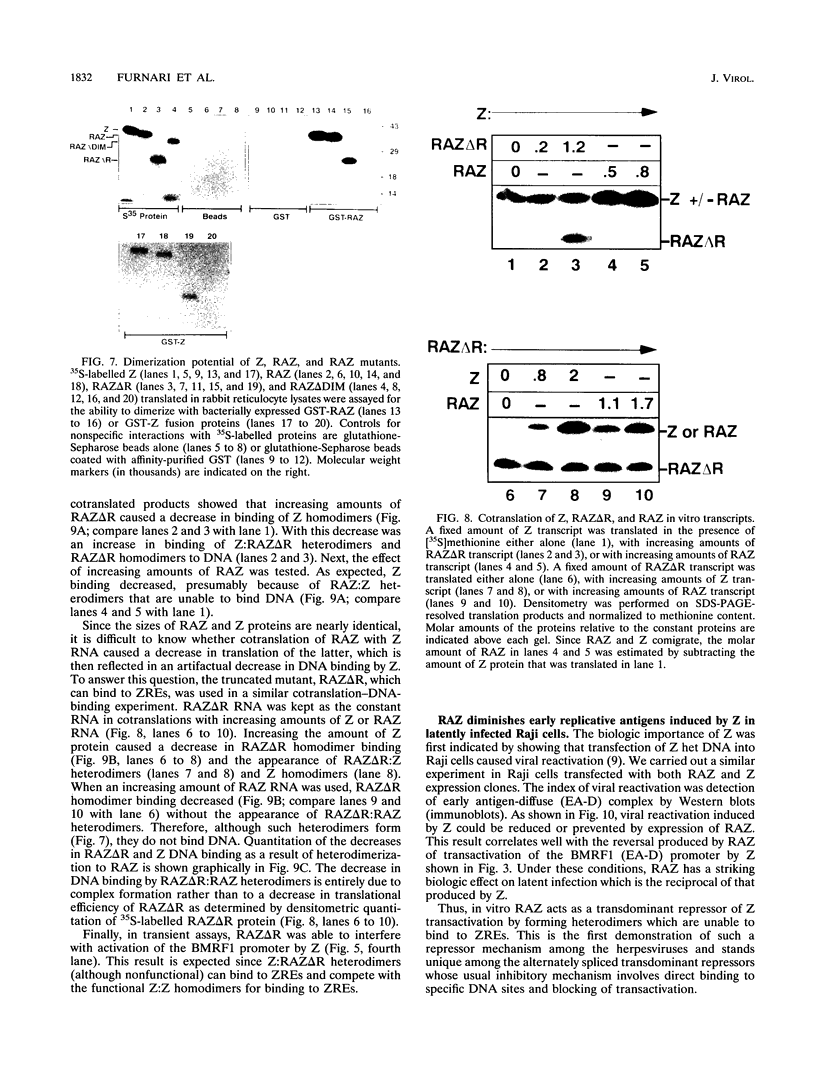
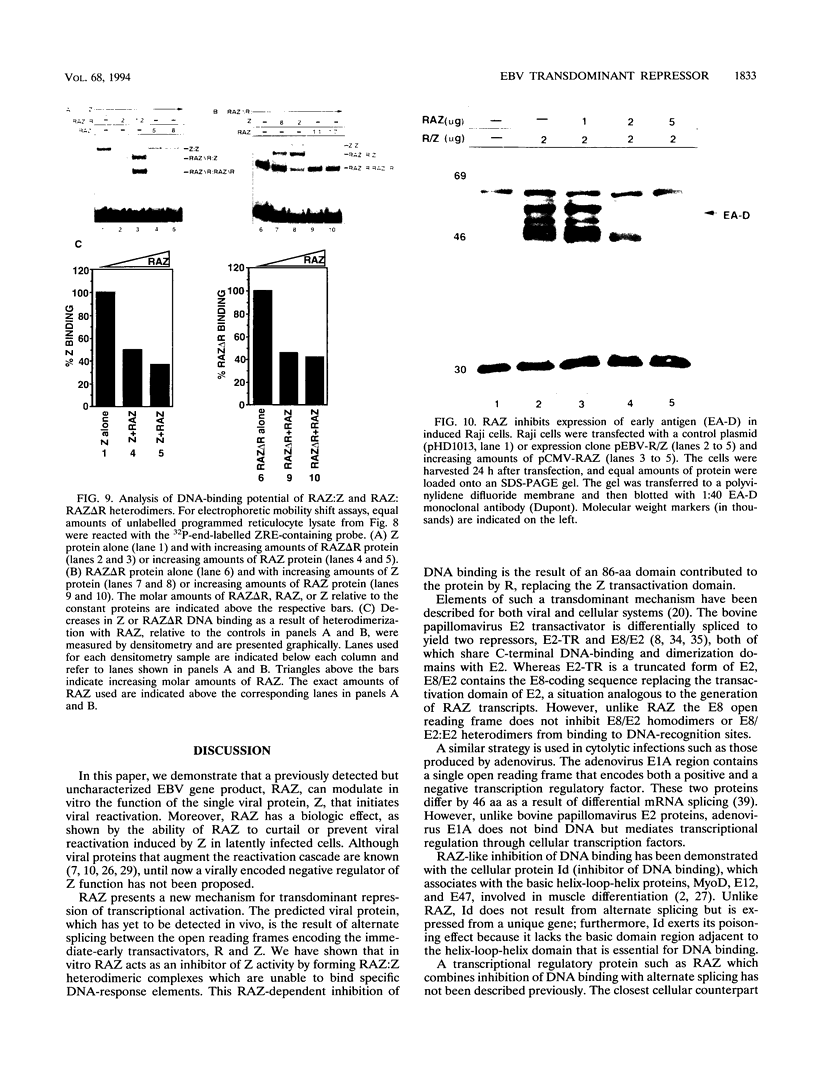
Epstein-Barr virus (EBV) is associated with the development of several types of human cancers and is an important cause of lymphomas in immunocompromised hosts. Expression of the EBV BZLF1 immediate-early gene product (Z) triggers disruption of latency in EBV-infected cells. Z is a member of the b-Zip family of proteins and binds to AP-1-like sites in early viral promoters. Here we show that a viral RNA related to Z, in which there is replacement of the transactivation domain of Z by fusion through alternate splicing with a portion of another EBV transactivator, BRLF1 (R), can repress Z function. This differentially spliced mRNA is predicted to express a novel chimeric protein which we call RAZ for R and Z. RAZ retains the dimerization and DNA-binding domains of Z but loses its transactivation domain. We show that in vitro the RAZ protein acts transdominantly to repress transactivation of early promoters by Z. Repression is produced by dimerization of RAZ with Z resulting in RAZ:Z heterodimers that can no longer bind to Z-binding sites despite retention of the DNA-binding domains in both proteins. Deletion of the R domain of RAZ restores the ability of the truncated RAZ homodimers and RAZ:Z heterodimers to bind to DNA. A biologic effect of RAZ was shown by cotransfection of latently infected Raji cells with Z and RAZ expression clones; RAZ diminished viral reactivation induced by Z, as indicated by amount of early replicative antigens (EA-D) detected. The RAZ protein presents a model for transcriptional control unique among the herpesvirus and distinct from analogous viral and cellular repressors. RAZ, by limiting the availability of Z protein, is likely to modulate EBV reactivation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lassar A., Tapscott S., Thayer M., Lockshon D., Weintraub H. Id: a negative regulator of helix-loop-helix DNA binding proteins. Control of terminal myogenic differentiation. Ann N Y Acad Sci. 1990;599:1–11. doi: 10.1111/j.1749-6632.1990.tb42359.x. [DOI] [PubMed] [Google Scholar]
- Biggin M., Bodescot M., Perricaudet M., Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987 Oct;61(10):3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M., Kolman J., Katz D. A., Gradoville L., Barberis L., Miller G. Transcriptional synergy by the Epstein-Barr virus transactivator ZEBRA. J Virol. 1992 Aug;66(8):4803–4813. doi: 10.1128/jvi.66.8.4803-4813.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. N., Dong D. L., Hayward G. S., Hayward S. D. The Epstein-Barr virus Zta transactivator: a member of the bZIP family with unique DNA-binding specificity and a dimerization domain that lacks the characteristic heptad leucine zipper motif. J Virol. 1990 Jul;64(7):3358–3369. doi: 10.1128/jvi.64.7.3358-3369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P., Gruffat H., Chevallier-Greco A., Buisson M., Sergeant A. The Epstein-Barr virus (EBV) early promoter DR contains a cis-acting element responsive to the EBV transactivator EB1 and an enhancer with constitutive and inducible activities. J Virol. 1989 Feb;63(2):607–614. doi: 10.1128/jvi.63.2.607-614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J., Vaillancourt P., Stenlund A., Botchan M. Bovine papillomavirus type 1 encodes two forms of a transcriptional repressor: structural and functional analysis of new viral cDNAs. J Virol. 1989 Apr;63(4):1743–1755. doi: 10.1128/jvi.63.4.1743-1755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M. A., Leahy J., Hardwick J. M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990 Jan;64(1):313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daibata M., Humphreys R. E., Takada K., Sairenji T. Activation of latent EBV via anti-IgG-triggered, second messenger pathways in the Burkitt's lymphoma cell line Akata. J Immunol. 1990 Jun 15;144(12):4788–4793. [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Davis M. G., Kenney S. C., Kamine J., Pagano J. S., Huang E. S. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrazanski P., Noguchi T., Kovary K., Rizzo C. A., Lazo P. S., Bravo R. Both products of the fosB gene, FosB and its short form, FosB/SF, are transcriptional activators in fibroblasts. Mol Cell Biol. 1991 Nov;11(11):5470–5478. doi: 10.1128/mcb.11.11.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Rowe D. T., Rooney C. M., Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989 Jan;8(1):127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E. K., Borras A. M., Lytle J. P., Speck S. H. Characterization of the Epstein-Barr virus BZLF1 protein transactivation domain. J Virol. 1992 Feb;66(2):922–929. doi: 10.1128/jvi.66.2.922-929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E. K., Goldfeld A. E., Speck S. H. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J Virol. 1991 Dec;65(12):7073–7077. doi: 10.1128/jvi.65.12.7073-7077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E., Speck S. H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990 Mar;64(3):1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Furnari F. B., Adams M. D., Pagano J. S. Regulation of the Epstein-Barr virus DNA polymerase gene. J Virol. 1992 May;66(5):2837–2845. doi: 10.1128/jvi.66.5.2837-2845.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruffat H., Manet E., Rigolet A., Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Dec 11;18(23):6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley-Guthrie E. A., Quinlivan E. B., Mar E. C., Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990 Aug;64(8):3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jen Y., Weintraub H., Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992 Aug;6(8):1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- Kenney S. C., Holley-Guthrie E., Quinlivan E. B., Gutsch D., Zhang Q., Bender T., Giot J. F., Sergeant A. The cellular oncogene c-myb can interact synergistically with the Epstein-Barr virus BZLF1 transactivator in lymphoid cells. Mol Cell Biol. 1992 Jan;12(1):136–146. doi: 10.1128/mcb.12.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Holley-Guthrie E., Lin J. C., Mar E. C., Pagano J. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J Virol. 1989 Apr;63(4):1729–1736. doi: 10.1128/jvi.63.4.1729-1736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Holley-Guthrie E., Mar E. C., Lin J. C., Markovitz D., Pagano J. The Epstein-Barr virus immediate-early gene product, BMLF1, acts in trans by a posttranscriptional mechanism which is reporter gene dependent. J Virol. 1989 Sep;63(9):3870–3877. doi: 10.1128/jvi.63.9.3870-3877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Packham G., Cook A., Farrell P. J. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991 Feb;6(2):195–204. [PubMed] [Google Scholar]
- Kwong A. D., Frenkel N. Herpes simplex virus-infected cells contain a function(s) that destabilizes both host and viral mRNAs. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1926–1930. doi: 10.1073/pnas.84.7.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Hubbert N. L., Howley P. M., Schiller J. T. Genetic assignment of multiple E2 gene products in bovine papillomavirus-transformed cells. J Virol. 1989 Jul;63(7):3151–3154. doi: 10.1128/jvi.63.7.3151-3154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990 Jun;64(6):2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. The Zta trans-activator protein stabilizes TFIID association with promoter DNA by direct protein-protein interaction. Genes Dev. 1991 Dec;5(12B):2441–2454. doi: 10.1101/gad.5.12b.2441. [DOI] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Hayward S. D. Responsiveness of the Epstein-Barr virus NotI repeat promoter to the Z transactivator is mediated in a cell-type-specific manner by two independent signal regions. J Virol. 1989 Jul;63(7):3040–3050. doi: 10.1128/jvi.63.7.3040-3050.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Manet E., Gruffat H., Trescol-Biemont M. C., Moreno N., Chambard P., Giot J. F., Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989 Jun;8(6):1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manet E., Rigolet A., Gruffat H., Giot J. F., Sergeant A. Domains of the Epstein-Barr virus (EBV) transcription factor R required for dimerization, DNA binding and activation. Nucleic Acids Res. 1991 May 25;19(10):2661–2667. doi: 10.1093/nar/19.10.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. The switch between latency and replication of Epstein-Barr virus. J Infect Dis. 1990 May;161(5):833–844. doi: 10.1093/infdis/161.5.833. [DOI] [PubMed] [Google Scholar]
- Muller M. T. Binding of the herpes simplex virus immediate-early gene product ICP4 to its own transcription start site. J Virol. 1987 Mar;61(3):858–865. doi: 10.1128/jvi.61.3.858-865.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg D., Lucibello F. C., Schuermann M., Müller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 1991 Jul;5(7):1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991 Feb 22;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packham G., Economou A., Rooney C. M., Rowe D. T., Farrell P. J. Structure and function of the Epstein-Barr virus BZLF1 protein. J Virol. 1990 May;64(5):2110–2116. doi: 10.1128/jvi.64.5.2110-2116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. S., Boundy A., O'Hare P., Pizzorno M. C., Ciufo D. M., Hayward G. S. Direct correlation between a negative autoregulatory response element at the cap site of the herpes simplex virus type 1 IE175 (alpha 4) promoter and a specific binding site for the IE175 (ICP4) protein. J Virol. 1988 Nov;62(11):4307–4320. doi: 10.1128/jvi.62.11.4307-4320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben S. M., Klement J. F., Coleman T. A., Maher M., Chen C. H., Rosen C. A. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992 May;6(5):745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- Sista N. D., Pagano J. S., Liao W., Kenney S. Retinoic acid is a negative regulator of the Epstein-Barr virus protein (BZLF1) that mediates disruption of latent infection. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3894–3898. doi: 10.1073/pnas.90.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J., Wisdom R. M., Tratner I., Verma I. M. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]