Abstract
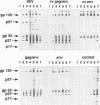
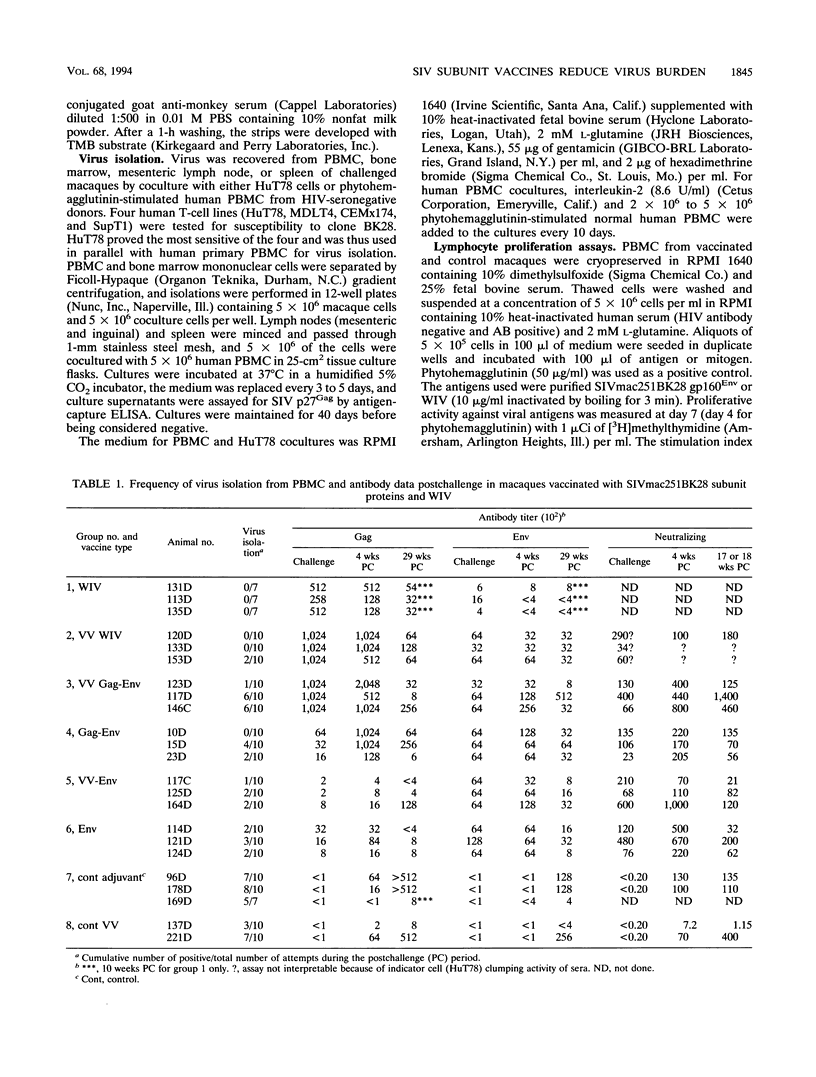
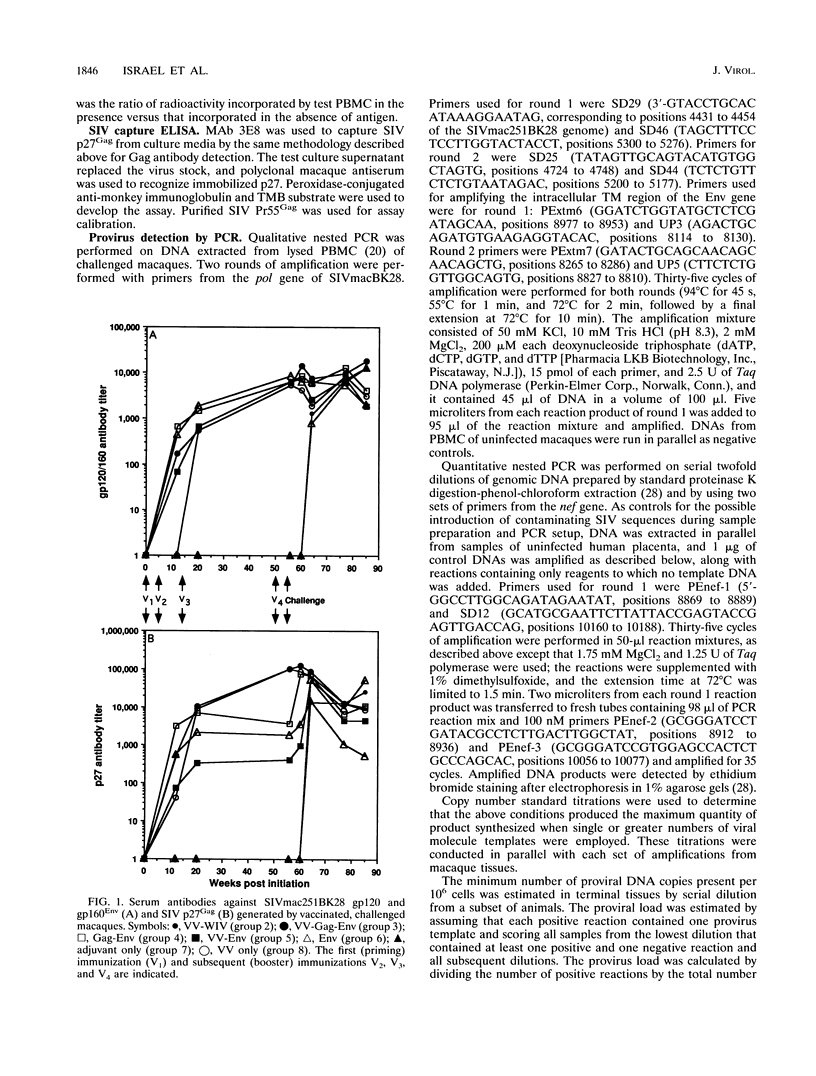
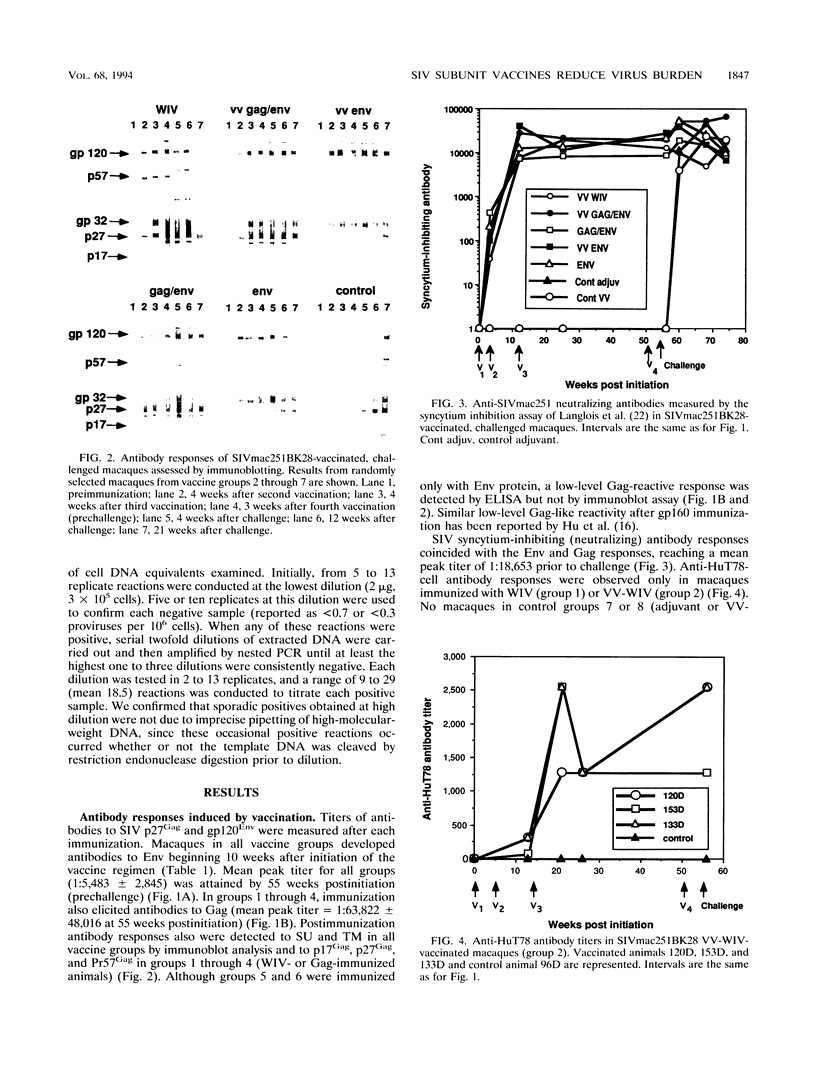
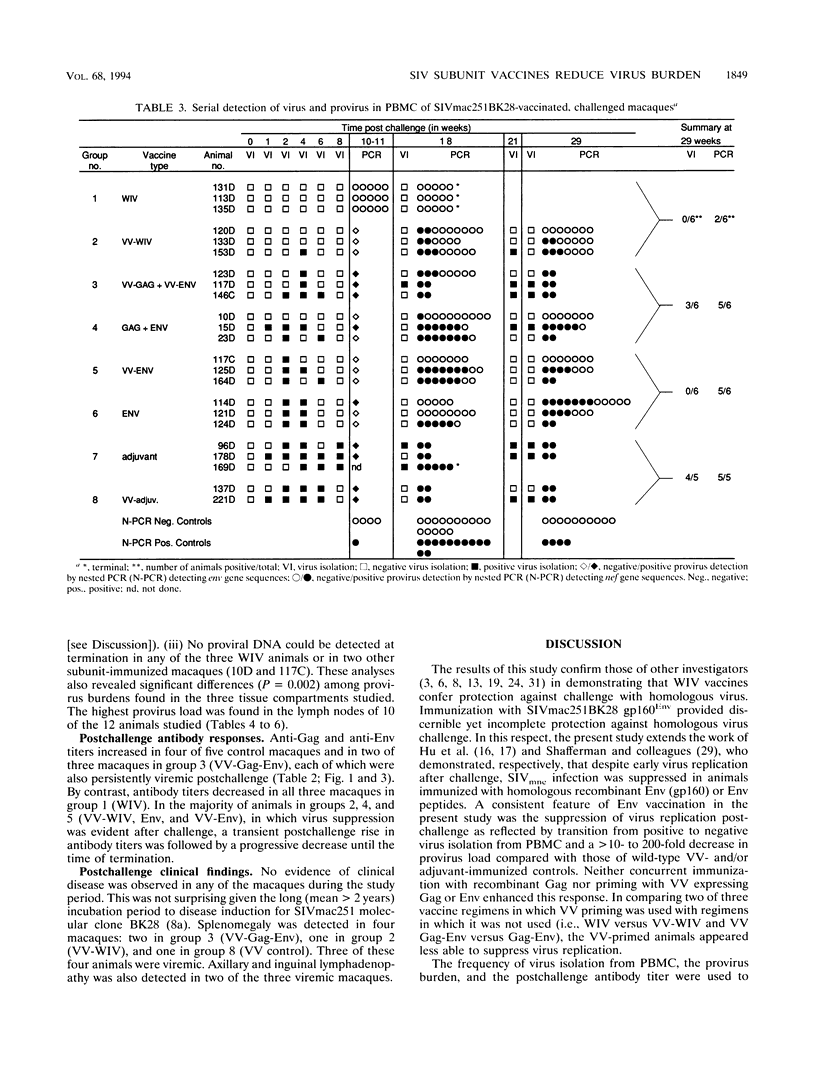
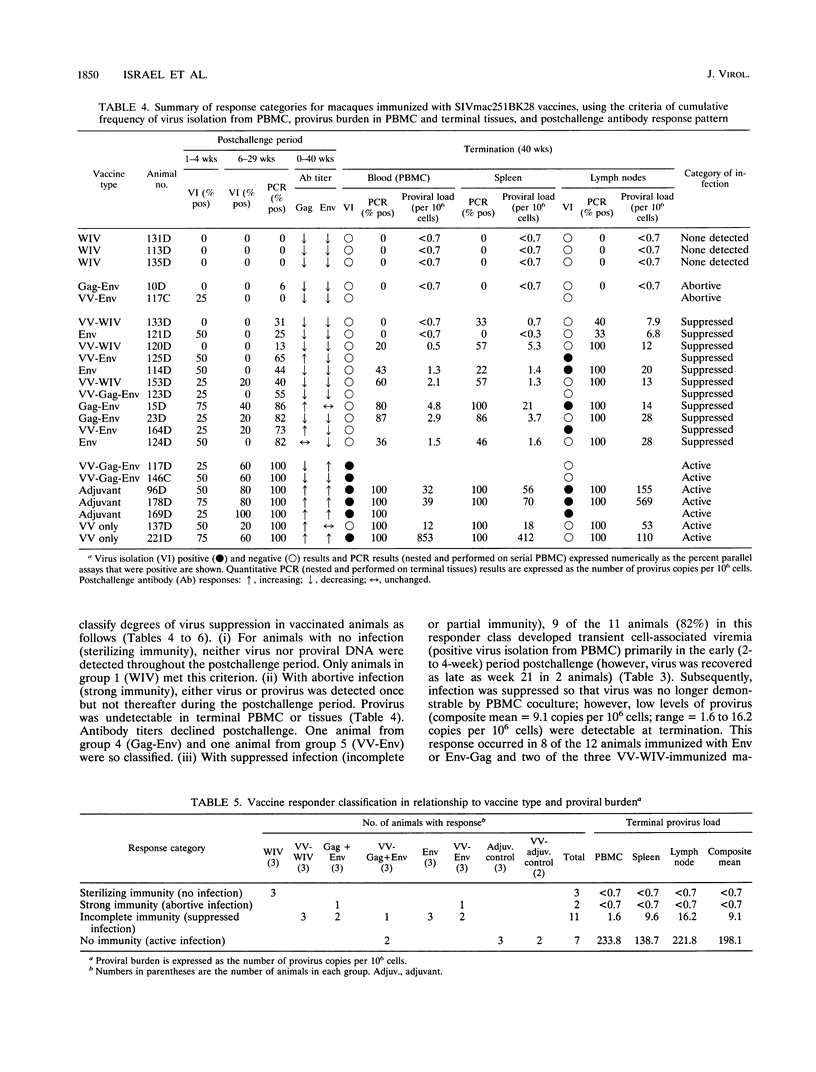
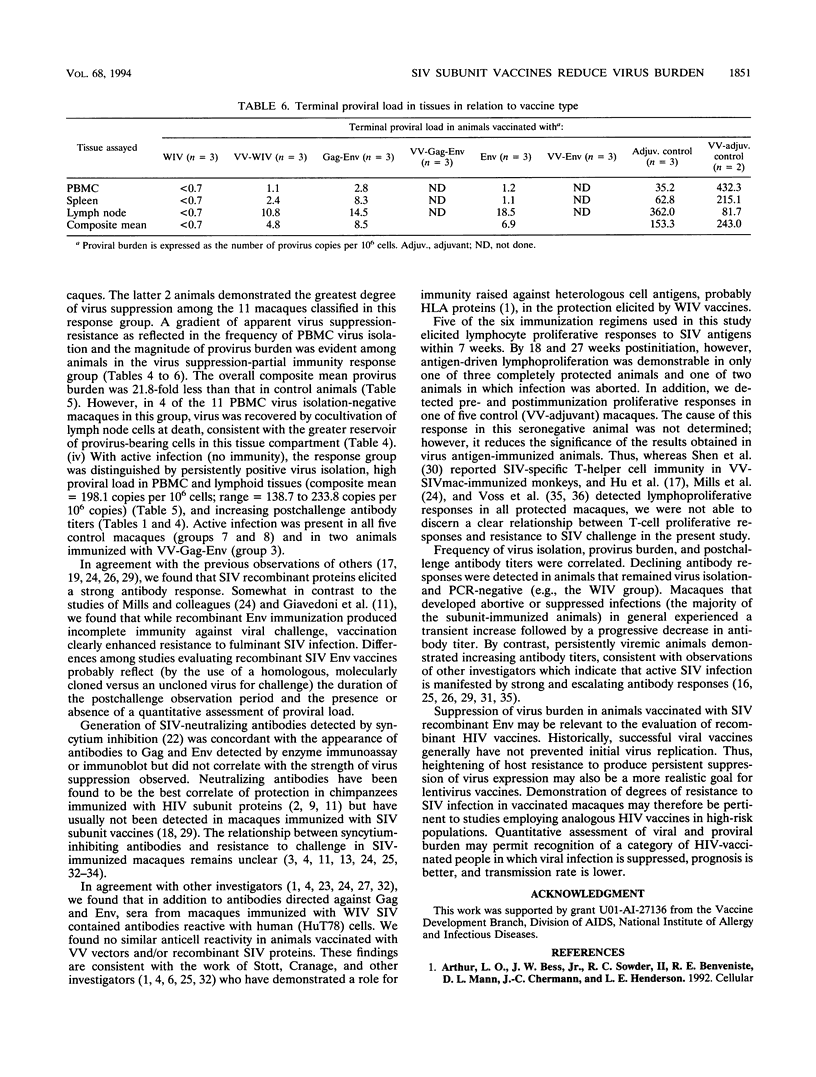
We compared the efficacy of immunization with either simian immunodeficiency virus (SIV) Env glycoprotein (Env), Env plus Gag proteins (Gag-Env), or whole inactivated virus (WIV), with or without recombinant live vaccinia vector (VV) priming, in protecting 23 rhesus macaques (six vaccine and two control groups) from challenge with SIVmac251 clone BK28. Vaccination elicited high titers of syncytium-inhibiting and anti-Env (gp120/gp160) antibodies in all vaccinated macaques and anti-Gag (p27) antibodies in groups immunized with WIV or Gag-Env. Only WIV-immunized macaques developed anticell (HuT78) antibodies. After homologous low-dose intravenous virus challenge, we used frequency of virus isolation, provirus burden, and change in antibody titers to define four levels of resistance to SIV infection as follows. (i) No infection ("sterilizing" immunity) was induced only in WIV-immunized animals. (ii) Abortive infection (strong immunity) was defined when virus or provirus were detected early in the postchallenge period but not thereafter and no evidence of virus or provirus was detected in terminal tissues. This response was observed in two animals (one VV-Env and one Gag-Env). (iii) Suppression of infection (incomplete or partial immunity) described a gradient of virus suppression manifested by termination of viremia, declining postchallenge antibody titers, and low levels (composite mean = 9.1 copies per 10(6) cells) of provirus detectable in peripheral blood mononuclear cells or lymphoid tissues at termination (40 weeks postchallenge). This response occurred in the majority (8 of 12) of subunit-vaccinated animals. (iv) Active infection (no immunity) was characterized by persistent virus isolation from blood mononuclear cells, increasing viral antibody titers postchallenge, and high levels (composite mean = 198 copies per 10(6) cells) of provirus in terminal tissues and blood. Active infection developed in all controls and two of three VV-Gag-Env-immunized animals. The results of this study restate the protective effect of inactivated whole virus vaccines produced in heterologous cells but more importantly demonstrate that a gradient of suppression of challenge virus growth, reflecting partial resistance to SIV infection, is induced by subunit vaccination. The latter finding may be pertinent to studies with human immunodeficiency virus vaccines, in which it is plausible that vaccination may elicit significant suppression of virus infection and pathogenicity rather than sterilizing immunity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., McGraw T. P., Keddie E., Yee J. L., Rosenthal A., Langlois A. J., Dickover R., Donovan R., Luciw P. A., Jennings M. B. Vaccine protection of rhesus macaques against simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1239–1246. doi: 10.1089/aid.1990.6.1239. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Polyanskaya N., McBride B., Cook N., Ashworth L. A., Dennis M., Baskerville A., Greenaway P. J., Corcoran T., Kitchin P. Studies on the specificity of the vaccine effect elicited by inactivated simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1993 Jan;9(1):13–22. doi: 10.1089/aid.1993.9.13. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Delchambre M., Gheysen D., Thines D., Thiriart C., Jacobs E., Verdin E., Horth M., Burny A., Bex F. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 1989 Sep;8(9):2653–2660. doi: 10.1002/j.1460-2075.1989.tb08405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Wyand M. S., Kodama T., Ringler D. J., Arthur L. O., Sehgal P. K., Letvin N. L., King N. W., Daniel M. D. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emini E. A., Nara P. L., Schleif W. A., Lewis J. A., Davide J. P., Lee D. R., Kessler J., Conley S., Matsushita S., Putney S. D. Antibody-mediated in vitro neutralization of human immunodeficiency virus type 1 abolishes infectivity for chimpanzees. J Virol. 1990 Aug;64(8):3674–3678. doi: 10.1128/jvi.64.8.3674-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., Nara P., Barre-Sinoussi F., Chaput A., Greenberg M. L., Muchmore E., Kieny M. P., Girard M. Vaccine protection of chimpanzees against challenge with HIV-1-infected peripheral blood mononuclear cells. Science. 1992 Jun 19;256(5064):1687–1690. doi: 10.1126/science.256.5064.1687. [DOI] [PubMed] [Google Scholar]
- Giavedoni L. D., Planelles V., Haigwood N. L., Ahmad S., Kluge J. D., Marthas M. L., Gardner M. B., Luciw P. A., Yilma T. D. Immune response of rhesus macaques to recombinant simian immunodeficiency virus gp130 does not protect from challenge infection. J Virol. 1993 Jan;67(1):577–583. doi: 10.1128/jvi.67.1.577-583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung S., Norley S. G., Ennen J., Cichutek K., Plesker R., Kurth R. Vaccine protection against SIVmac infection by high- but not low-dose whole inactivated virus immunogen. J Acquir Immune Defic Syndr. 1992;5(5):461–468. [PubMed] [Google Scholar]
- Hirsch V. M., Edmondson P., Murphey-Corb M., Arbeille B., Johnson P. R., Mullins J. I. SIV adaptation to human cells. Nature. 1989 Oct 19;341(6243):573–574. doi: 10.1038/341573a0. [DOI] [PubMed] [Google Scholar]
- Horth M., Lambrecht B., Khim M. C., Bex F., Thiriart C., Ruysschaert J. M., Burny A., Brasseur R. Theoretical and functional analysis of the SIV fusion peptide. EMBO J. 1991 Oct;10(10):2747–2755. doi: 10.1002/j.1460-2075.1991.tb07823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Abrams K., Barber G. N., Moran P., Zarling J. M., Langlois A. J., Kuller L., Morton W. R., Benveniste R. E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992 Jan 24;255(5043):456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Abrams K., Misher L., Stallard V., Moran P., Zarling J. M., Langlois A. J., Kuller L., Morton W. R., Benveniste R. E. Evaluation of protective efficacy of recombinant subunit vaccines against simian immunodeficiency virus infection of macaques. J Med Primatol. 1992 Feb-May;21(2-3):119–125. [PubMed] [Google Scholar]
- Johnson P. R., Montefiori D. C., Goldstein S., Hamm T. E., Zhou J., Kitov S., Haigwood N. L., Misher L., London W. T., Gerin J. L. Inactivated whole SIV vaccine in macaques: evaluation of protective efficacy against challenge with cell-free virus or infected cells. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1501–1505. doi: 10.1089/aid.1992.8.1501. [DOI] [PubMed] [Google Scholar]
- Johnson P. R., Montefiori D. C., Goldstein S., Hamm T. E., Zhou J., Kitov S., Haigwood N. L., Misher L., London W. T., Gerin J. L. Inactivated whole-virus vaccine derived from a proviral DNA clone of simian immunodeficiency virus induces high levels of neutralizing antibodies and confers protection against heterologous challenge. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2175–2179. doi: 10.1073/pnas.89.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois A. J., Weinhold K. J., Matthews T. J., Bolognesi D. P. In vitro assays for detecting neutralizing and fusion-inhibiting antibodies to SIVMAC251. AIDS Res Hum Retroviruses. 1991 Aug;7(8):713–720. doi: 10.1089/aid.1991.7.713. [DOI] [PubMed] [Google Scholar]
- Langlois A. J., Weinhold K. J., Matthews T. J., Greenberg M. L., Bolognesi D. P. The ability of certain SIV vaccines to provoke reactions against normal cells. Science. 1992 Jan 17;255(5042):292–293. doi: 10.1126/science.1549775. [DOI] [PubMed] [Google Scholar]
- Le Grand R., Vaslin B., Vogt G., Roques P., Humbert M., Dormont D. AIDS vaccine developments. Nature. 1992 Feb 20;355(6362):684–684. doi: 10.1038/355684a0. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Davison-Fairburn B., Montelaro R. C., Miller M., West M., Ohkawa S., Baskin G. B., Zhang J. Y., Putney S. D. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989 Dec 8;246(4935):1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Montelaro R. C., Miller M. A., West M., Martin L. N., Davison-Fairburn B., Ohkawa S., Baskin G. B., Zhang J. Y., Miller G. B. Efficacy of SIV/deltaB670 glycoprotein-enriched and glycoprotein-depleted subunit vaccines in protecting against infection and disease in rhesus monkeys. AIDS. 1991 Jun;5(6):655–662. doi: 10.1097/00002030-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Osterhaus A., de Vries P., Heeney J. AIDS vaccine developments. Nature. 1992 Feb 20;355(6362):684–685. doi: 10.1038/355684b0. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Jahrling P. B., Benveniste R. E., Lewis M. G., Phipps T. J., Eden-McCutchan F., Sadoff J., Eddy G. A., Burke D. S. Protection of macaques with a simian immunodeficiency virus envelope peptide vaccine based on conserved human immunodeficiency virus type 1 sequences. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7126–7130. doi: 10.1073/pnas.88.16.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Chen Z. W., Miller M. D., Stallard V., Mazzara G. P., Panicali D. L., Letvin N. L. Recombinant virus vaccine-induced SIV-specific CD8+ cytotoxic T lymphocytes. Science. 1991 Apr 19;252(5004):440–443. doi: 10.1126/science.1708168. [DOI] [PubMed] [Google Scholar]
- Stahl-Hennig C., Voss G., Nick S., Petry H., Fuchs D., Wachter H., Coulibaly C., Lüke W., Hunsmann G. Immunization with tween-ether-treated SIV adsorbed onto aluminum hydroxide protects monkeys against experimental SIV infection. Virology. 1992 Feb;186(2):588–596. doi: 10.1016/0042-6822(92)90025-k. [DOI] [PubMed] [Google Scholar]
- Stott E. J. Anti-cell antibody in macaques. Nature. 1991 Oct 3;353(6343):393–393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Sutjipto S., Pedersen N. C., Miller C. J., Gardner M. B., Hanson C. V., Gettie A., Jennings M., Higgins J., Marx P. A. Inactivated simian immunodeficiency virus vaccine failed to protect rhesus macaques from intravenous or genital mucosal infection but delayed disease in intravenously exposed animals. J Virol. 1990 May;64(5):2290–2297. doi: 10.1128/jvi.64.5.2290-2297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss G., Nick S., Stahl-Hennig C., Coulibaly C., Petry H., Lüke W., Hunsmann G. Potential significance of the cellular immune response against the macaque strain of simian immunodeficiency virus (SIVMAC) in immunized and infected rhesus macaques. J Gen Virol. 1992 Sep;73(Pt 9):2273–2281. doi: 10.1099/0022-1317-73-9-2273. [DOI] [PubMed] [Google Scholar]
- Voss G., Stahl-Hennig C., Petry H., Coulibaly C., Nick S., Fuchs D., Wachter H., Lüke W., Hunsmann G. Immunization of rhesus monkeys with high- and low-dose Tween-ether-disrupted SIVMAC. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1397–1400. doi: 10.1089/aid.1992.8.1397. [DOI] [PubMed] [Google Scholar]