Abstract
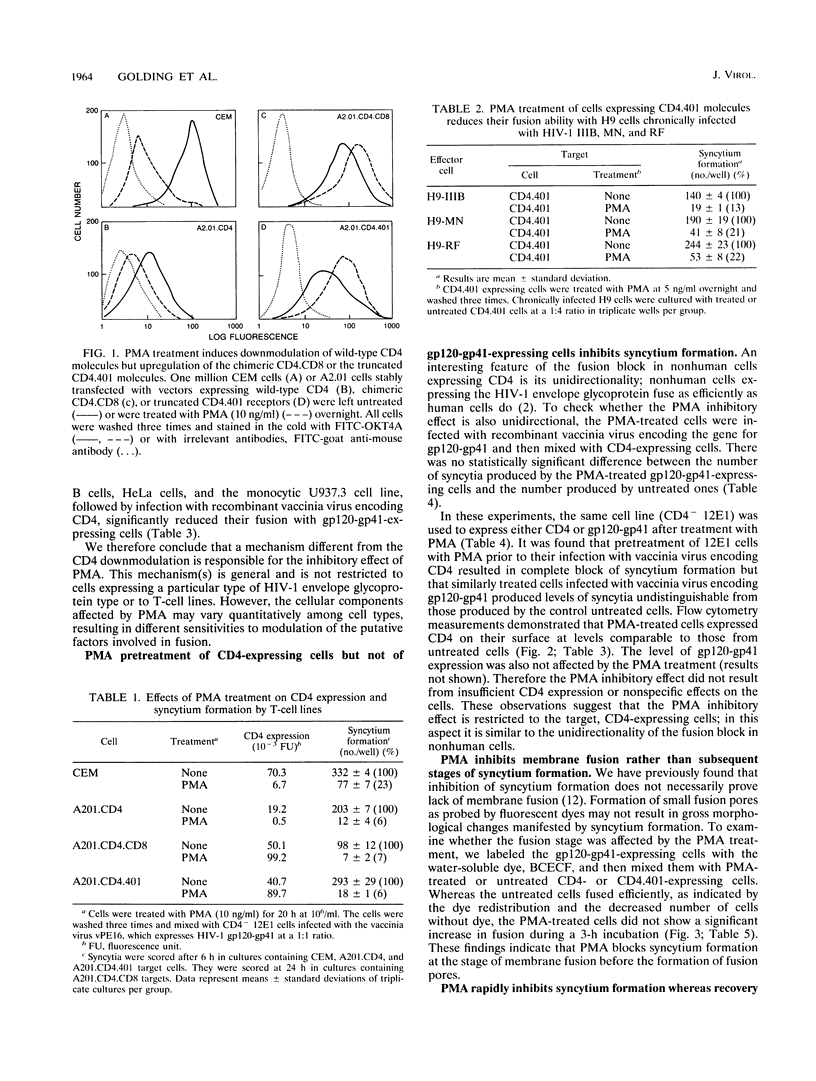
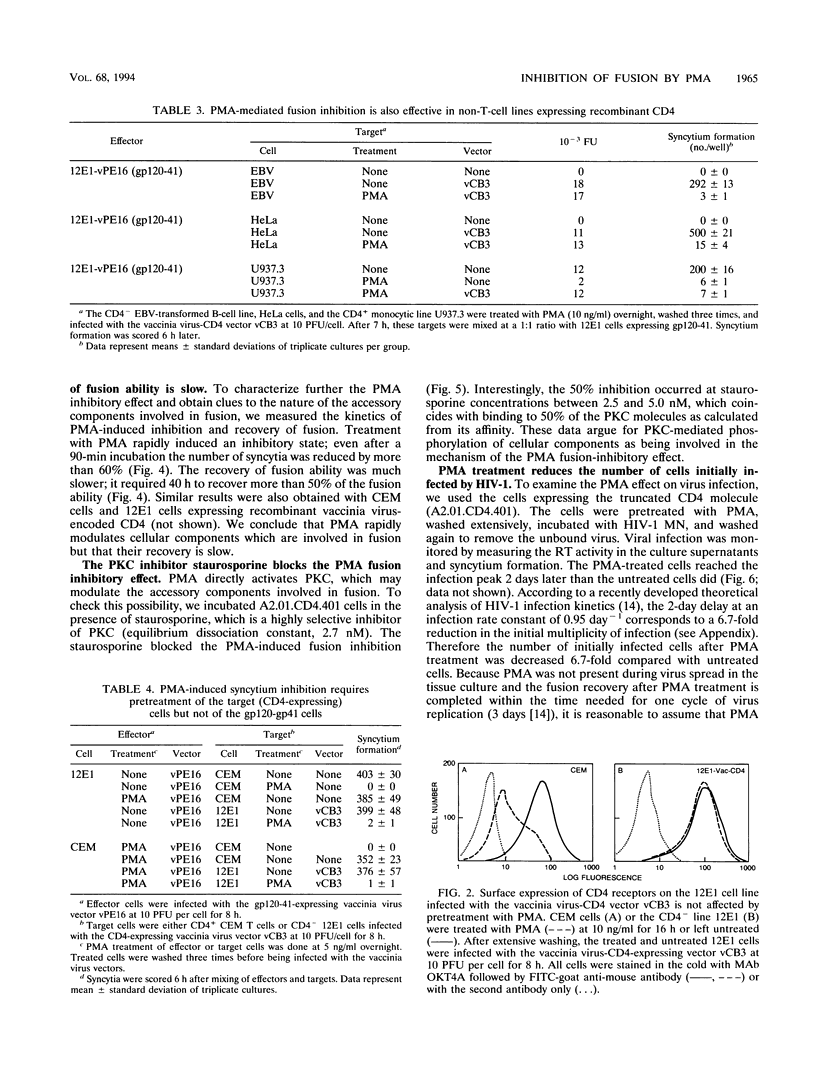
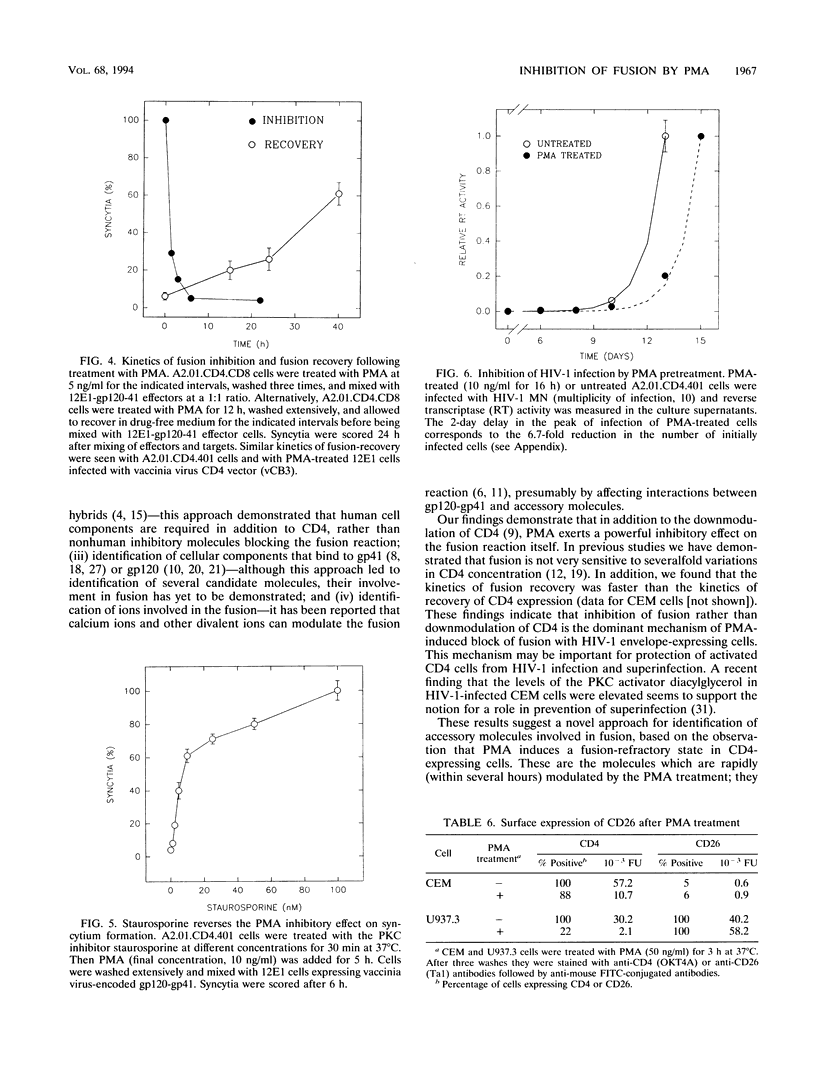
The phorbol ester phorbol myristate acetate (PMA) strongly inhibits human immunodeficiency virus type 1 (HIV-1)-induced syncytium formation; it has been suggested that this inhibitory effect is due to the transient downmodulation of the surface-associated CD4 receptors by PMA (I. H. Chowdhury, Y. Koyanagi, S. Kobayashi, Y. Hamamoto, H. Yoshiyama, T. Yoshida, and N. Yamamoto, Virology 176:126-132, 1990). Surprisingly, PMA treatment of cells expressing truncated (A2.01.CD4.401) and hybrid (A2.01.CD4.CD8) CD4 molecules, which are not downmodulated (P. Bedinger, A. Moriarty, R. C. von Borstel II, N. J. Donovan, K. S. Steimer, and D. R. Littman, Nature [London] 334:162-165, 1988), inhibited their fusion with CD4- (12E1) cells expressing vaccinia virus-encoded HIV-1 envelope glycoprotein (gp120-gp41) and with chronically HIV-1-infected H9 (MN, IIIB, or RF) cells. PMA pretreatment of T (12E1) and non-T (HeLa, U937.3, and Epstein-Barr virus-transformed B) cell lines expressing vaccinia virus-encoded CD4 also blocked fusion with 12E1 cells expressing vaccinia virus-encoded gp120-gp41. Interestingly, pretreatment of the gp120-gp41-expressing 12E1 cells with PMA did not alter their fusion with untreated CD4-expressing cells. Although the inhibitory effect of PMA was rapid and treatment for 1.5 h with 5 ng of PMA per ml was sufficient to reduce fusion by more than 50%, the recovery after treatment was slow and more than 40 h was needed before the cells regained half of their fusion potential. The inhibitory effect of PMA was blocked by staurosporine in a dose-dependent fashion, suggesting that it is mediated by protein kinase C. PMA treatment of A2.01.CD4.401 cells reduced the number of infected cells 6.7-fold, as estimated by a quantitative analysis of the HIV-1 MN infection kinetics, probably by affecting the stage of virus entry into cells. CD26 surface expression was not significantly changed by PMA treatment. We conclude that PMA inhibits the CD4-gp120-gp41-mediated fusion by modulating an accessory component(s), different from CD26, in the target CD4-expressing cells. These findings suggest a novel approach for identification of accessory molecules involved in fusion and may have implications for the development of antiviral agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoki N., Shioda T., Satoh H., Shibuta H. Syncytium formation of human and non-human cells by recombinant vaccinia viruses carrying the HIV env gene and human CD4 gene. AIDS. 1991 Jul;5(7):871–875. doi: 10.1097/00002030-199107000-00012. [DOI] [PubMed] [Google Scholar]
- Ashorn P. A., Berger E. A., Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990 May;64(5):2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedinger P., Moriarty A., von Borstel R. C., 2nd, Donovan N. J., Steimer K. S., Littman D. R. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988 Jul 14;334(6178):162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- Broder C. C., Berger E. A. CD4 molecules with a diversity of mutations encompassing the CDR3 region efficiently support human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion. J Virol. 1993 Feb;67(2):913–926. doi: 10.1128/jvi.67.2.913-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder C. C., Dimitrov D. S., Blumenthal R., Berger E. A. The block to HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s). Virology. 1993 Mar;193(1):483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- Busso M., Thornthwaite J., Resnick L. HIV-induced syncytium formation requires the formation of conjugates between virus-infected and uninfected T-cells in vitro. AIDS. 1991 Dec;5(12):1425–1432. doi: 10.1097/00002030-199112000-00003. [DOI] [PubMed] [Google Scholar]
- Callebaut C., Krust B., Jacotot E., Hovanessian A. G. T cell activation antigen, CD26, as a cofactor for entry of HIV in CD4+ cells. Science. 1993 Dec 24;262(5142):2045–2050. doi: 10.1126/science.7903479. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Ebenbichler C., Vornhagen R., Schulz T. F., Steindl F., Böck G., Katinger H., Dierich M. P. HIV-1 gp41 contains two sites for interaction with several proteins on the helper T-lymphoid cell line, H9. AIDS. 1992 Jun;6(6):533–539. doi: 10.1097/00002030-199206000-00002. [DOI] [PubMed] [Google Scholar]
- Chowdhury I. H., Koyanagi Y., Kobayashi S., Hamamoto Y., Yoshiyama H., Yoshida T., Yamamoto N. The phorbol ester TPA strongly inhibits HIV-1-induced syncytia formation but enhances virus production: possible involvement of protein kinase C pathway. Virology. 1990 May;176(1):126–132. doi: 10.1016/0042-6822(90)90237-l. [DOI] [PubMed] [Google Scholar]
- Clements G. J., Price-Jones M. J., Stephens P. E., Sutton C., Schulz T. F., Clapham P. R., McKeating J. A., McClure M. O., Thomson S., Marsh M. The V3 loops of the HIV-1 and HIV-2 surface glycoproteins contain proteolytic cleavage sites: a possible function in viral fusion? AIDS Res Hum Retroviruses. 1991 Jan;7(1):3–16. doi: 10.1089/aid.1991.7.3. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Broder C. C., Berger E. A., Blumenthal R. Calcium ions are required for cell fusion mediated by the CD4-human immunodeficiency virus type 1 envelope glycoprotein interaction. J Virol. 1993 Mar;67(3):1647–1652. doi: 10.1128/jvi.67.3.1647-1652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Golding H., Blumenthal R. Initial stages of HIV-1 envelope glycoprotein-mediated cell fusion monitored by a new assay based on redistribution of fluorescent dyes. AIDS Res Hum Retroviruses. 1991 Oct;7(10):799–805. doi: 10.1089/aid.1991.7.799. [DOI] [PubMed] [Google Scholar]
- Dimitrov D. S., Hillman K., Manischewitz J., Blumenthal R., Golding H. Kinetics of soluble CD4 binding to cells expressing human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1992 Jan;66(1):132–138. doi: 10.1128/jvi.66.1.132-138.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov D. S., Willey R. L., Sato H., Chang L. J., Blumenthal R., Martin M. A. Quantitation of human immunodeficiency virus type 1 infection kinetics. J Virol. 1993 Apr;67(4):2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T., Charneau P., Clavel F., Alizon M. Complementation of murine cells for human immunodeficiency virus envelope/CD4-mediated fusion in human/murine heterokaryons. J Virol. 1992 Aug;66(8):4794–4802. doi: 10.1128/jvi.66.8.4794-4802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl P. L., Koenig S., Moss B. Biological and immunological properties of human immunodeficiency virus type 1 envelope glycoprotein: analysis of proteins with truncations and deletions expressed by recombinant vaccinia viruses. J Virol. 1991 Jan;65(1):31–41. doi: 10.1128/jvi.65.1.31-41.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe C., Beck A., Gelderblom H. R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3(10):965–974. [PubMed] [Google Scholar]
- Henderson L. A., Qureshi M. N. A peptide inhibitor of human immunodeficiency virus infection binds to novel human cell surface polypeptides. J Biol Chem. 1993 Jul 15;268(20):15291–15297. [PubMed] [Google Scholar]
- Hillman K., Shapira-Nahor O., Gruber M. F., Hooley J., Manischewitz J., Seeman R., Vujcic L., Geyer S. J., Golding H. Chemically induced CD4 mutants of a human T cell line. Evidence for dissociation between binding of HIV I envelope and susceptibility to HIV I infection and syncytia formation. J Immunol. 1990 Mar 15;144(6):2131–2139. [PubMed] [Google Scholar]
- Kido H., Fukutomi A., Katunuma N. A novel membrane-bound serine esterase in human T4(+)-lymphocytes is a binding protein of envelope glycoprotein gp120 of HIV-1. Biomed Biochim Acta. 1991;50(4-6):781–789. [PubMed] [Google Scholar]
- Kido H., Fukutomi A., Katunuma N. Tryptase TL2 in the membrane of human T4+ lymphocytes is a novel binding protein of the V3 domain of HIV-1 envelope glycoprotein gp 120. FEBS Lett. 1991 Jul 29;286(1-2):233–236. doi: 10.1016/0014-5793(91)80981-8. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Feinberg M. B., Reyes G. R., Rabin L., Banapour B., Chakrabarti S., Moss B., Wong-Staal F., Steimer K. S., Engleman E. G. Induction of CD4-dependent cell fusion by the HTLV-III/LAV envelope glycoprotein. Nature. 1986 Oct 23;323(6090):725–728. doi: 10.1038/323725a0. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Poncelet P., Carayon P. Cytofluorometric quantification of cell-surface antigens by indirect immunofluorescence using monoclonal antibodies. J Immunol Methods. 1985 Dec 17;85(1):65–74. doi: 10.1016/0022-1759(85)90274-1. [DOI] [PubMed] [Google Scholar]
- Poulin L., Evans L. A., Tang S. B., Barboza A., Legg H., Littman D. R., Levy J. A. Several CD4 domains can play a role in human immunodeficiency virus infection in cells. J Virol. 1991 Sep;65(9):4893–4901. doi: 10.1128/jvi.65.9.4893-4901.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi N. M., Coy D. H., Garry R. F., Henderson L. A. Characterization of a putative cellular receptor for HIV-1 transmembrane glycoprotein using synthetic peptides. AIDS. 1990 Jun;4(6):553–558. doi: 10.1097/00002030-199006000-00009. [DOI] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Gowda S. D., Lifson J. D., Penhallow R. C., Bensch K. G., Engleman E. G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987 Jun 5;49(5):659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- Tersmette M., van Dongen J. J., Clapham P. R., de Goede R. E., Wolvers-Tettero I. L., Geurts van Kessel A., Huisman J. G., Weiss R. A., Miedema F. Human immunodeficiency virus infection studied in CD4-expressing human-murine T-cell hybrids. Virology. 1989 Feb;168(2):267–273. doi: 10.1016/0042-6822(89)90266-3. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Matthews T. J., Bolognesi D. P., Bell R. M. Changes in bioactive lipids, alkylacylglycerol and ceramide, occur in HIV-infected cells. Biochem Biophys Res Commun. 1992 Aug 31;187(1):209–216. doi: 10.1016/s0006-291x(05)81480-9. [DOI] [PubMed] [Google Scholar]



