Abstract
We evaluated the ability of flagellin, a highly effective mucosal adjuvant in mice and non-human primates, to promote mucosal innate and adaptive immunity in aged mice. We found that intratracheal instillation of flagellin induced a stronger respiratory innate response in aged mice than in young mice, and that intranasal instillation of flagellin was equally effective at triggering recruitment of T and B lymphocytes to the draining lymph nodes of young and aged mice. Intranasal immunization of aged mice with flagellin and the Yersinia pestis protein F1 promoted specific IgG and IgA production, but at lower levels and lower avidities than in young mice. Although intranasal instillation of flagellin and F1 antigen increased germinal center formation and size in young mice, it did not do so in aged mice. Our findings are consistent with the conclusion that flagellin can promote adaptive immune responses in aged mice, but at a less robust level than in young mice.
Keywords: vaccination, mucosa, memory
1. Introduction
Vaccination is much less effective in elderly populations than in young populations (Goodwin et al., 2006). In humans, aged individuals have reduced numbers of antigen reactive CD4+ T lymphocytes following immunization (Kang et al., 2004) as well as reduced levels of cytokine production on a per cell basis (Deng et al., 2004). The ability of immunization to generate cytotoxic T lymphocyte (CTL) activity and antibody responses in aged individuals is also significantly less than in young individuals (Powers and Belshe, 1993). Numerous mechanisms contribute to these deficits including decreased IFN-γ signaling in macrophages (Yoon et al., 2004), expansion of CD4+CD28− (Weyand et al., 1998) and CD8+CD28− cell populations (Goronzy et al., 2001; Saurwein-Teissl et al., 2002), decreased cytoskeletal rearrangement (Garcia and Miller, 2002) and impaired intracellular signaling following stimulation (Miller, 1989; Tamir et al., 2000). The B cell compartment is also subject to age-related impairments including decreased expansion of antigen-specific B cells (Dailey et al., 2001) and decreased hypermutation within responding populations of antigen-specific B cells (van Dijk- Hard et al., 1997; Troutaud et al., 1999). Attrition of the naïve T cell population (Lazuardi et al., 2005) and diminished TCR diversity in the total T cell population (Naylor et al., 2005) compound the detrimental effects of the loss of naïve B cells (Johnson et al., 2002), decreased B cell lymphopoeisis (Miller and Allman, 2003) and reduced antibody repertoire in aged humans (Kolibab et al., 2005; Smithson et al., 2005). The combination of loss of function by aged cells and reduction in the repertoire of cells capable of responding to a given antigen impose severe limitations on the aged immune system.
However, results from studies with humans reveal that an enhanced immunization regimen can compensate for some of the defects inherent in the aged immune system. Increasing the antigen dose at immunization (Roos-Van Eijndhoven et al., 2001; Keitel et al., 2006) and increasing the number of immunizations (Roos-Van Eijndhoven et al., 2001) results in higher antibody titers in aged humans compared to standard immunization schedules. While administering more antigen is biologically effective at the level of the individual, production constraints (Dennis, 2006) limit the practicality of immunizing entire populations with higher antigen doses. Other immunomodulatory approaches such as administering inflammatory cytokines at the time of immunization have proven effective at enhancing responses in aged mice (Haynes et al., 2004) but are not being considered for inclusion in human vaccines. Currently alum is the only adjuvant employed for widespread use in humans (Petrovsky and Aguilar, 2004). Alum is moderately effective in young and middle-aged humans, though other adjuvants in development have been shown to be more effective in these age groups (Lewis et al., 2003). The development of new adjuvants for use in humans may provide additional means of compensating for age-related defects of the immune system and increasing vaccine efficacy in the elderly.
Flagellin, the major structural component of bacterial flagella (Lowy and Hanson, 1964; Lowy and McDonough, 1964) and the ligand for Toll Like Receptor 5 (TLR5) (Hayashi et al., 2001), has long been known to possess in vivo adjuvant activity (Waldmann and Munro, 1975). Its adjuvant activity is believed to result from its ability to promote dendritic cell maturation (McSorley et al., 2002; Means et al., 2003; Cuadros et al., 2004; Pino, 2005) and consequently facilitate antigen specific T cell proliferation (McSorley et al., 2002). Flagellin has also been shown to be an effective systemic and mucosal adjuvant in mice (McEwen et al., 1992; Levi and Arnon, 1996; Ben-Yedidia et al., 1999; Strindelius et al., 2004; Honko et al., 2006) and non-human primates (Honko et al., 2006). Additionally, intratracheal (i.t.) instillation of young mice with flagellin triggers a robust respiratory innate response (Honko and Mizel, 2004). Flagellin has been shown to be effective at promoting anti-viral immunity in aged mice (Ben-Yedidia et al., 1998). In view of flagellin’s efficacy as an adjuvant for immunization against bacterial (Newton et al., 1989; Honko et al., 2006), viral (McEwen et al., 1992; Levi and Arnon, 1996; Ben-Yedidia et al., 1998), and parasitic infections (Ben-Yedidia et al., 1999) in young mice, we have evaluated its ability to function as a Y. pestis vaccine adjuvant in aged mice.
Given the paucity of information on the adjuvant effect of flagellin in aged animals, we have examined key events in the development of the adaptive immune response in young and aged mice and determined how they differ. We have developed a Y. pestis vaccine (with flagellin as the adjuvant) which should soon enter Phase I clinical trials, and we anticipate that the results of our studies on aged mice will assist in shaping expectations of the ability of flagellin to function as an adjuvant in aged humans.
2. Materials and Methods
2.1 Mice
Eighteen months is a commonly accepted age for “aged” mice used for immunological studies (Tomer et al., 1991; Asanuma et al., 2001; Boehmer et al., 2004; Ehrchen et al., 2004; Mittler and Lee, 2004; Vannier et al., 2004; Alignani et al., 2005; El-Shaikh et al., 2006; Montes et al., 2006; Sen et al., 2006). For these studies, aged BALB/c mice from the Harlan colony were purchased through the National Institute on Aging, and 6 to 8 week-old BALB/c mice were purchased directly from Harlan (Indianapolis, IN). All mice were housed in the Wake Forest University Health Sciences animal facility in accordance with institutional and USDA regulations. All experiments were conducted in accordance with protocols approved by the Wake Forest University Health Sciences Animal Care and Use Committees.
2.2 Recombinant Proteins
The coding sequence for the F1 antigen of Yersinia pestis, caf1 (plasmid containing the entire caf operon kindly provided by J. B. Bliska, State University of New York, Stony Brook), was subcloned into the NdeI and XhoI sites of the pET29a expression vector from Novagen (EMD Biosciences, Inc., Madison, WI). Recombinant his-tagged Y. pestis F1 protein, Salmonella FliC flagellin, and the non-signaling FliC mutant 229 were produced as previously described (McDermott et al., 2000; Honko and Mizel, 2004; Honko et al., 2006). All recombinant proteins were purified using Acrodisc Mustang Q and E membranes (Pall Corporation, Ann Arbor, MI). Contaminating endotoxin levels were verified to be less than 10 pg LPS per µg protein by Limulus amebocyte lysate assay (Associates of Cape Cod, Inc., East Falmouth, MA).
2.3 Nonsurgical Intratracheal & Intranasal Instillation
Non-surgical i.t. instillation was conducted as previously described (Honko and Mizel, 2004). Forty µl was the standard volume used for all i.t. instillation. For intranasal (i.n.) instillations, 12 to 15 µl of solution were applied drop wise, alternating between nostrils. All immunizations with F1 protein were administered intranasally. For these experiments, flagellin and F1 were administered as separate proteins.
2.4 Bronchoalveolar Lavage & Nasal Wash
Mice were sacrificed by CO2 asphyxiation followed by cervical dislocation as a secondary method. Bronchoalveolar lavages (BAL) were conducted as previously described (Honko and Mizel, 2004), except that lungs were inflated only once with 1.5 to 2 ml of PBS. For nasal washes a similar procedure was followed except the tubing was inserted through an incision in the trachea toward the nasal passageway rather than toward the bronchi. Nasal passages were flushed with 0.35 ml of PBS, of which an average of 0.32 ml was recovered into a 1.5 ml tube held at the nose of the mouse. Bronchoalveolar lavage fluid (BALF) and nasal wash fluid were centrifuged to remove cells. Supernatant was stored at −70°C until analysis.
2.5 Cell counts and cytospins
Cells collected from BALF were counted in a hemacytometer. Two hundred microliters of cell suspension were dispersed onto slides using a cytospin machine set at 450 rpm for 5 min. Slides were stained using a Hema 3 stain set (Fisher Scientific Co.) according to the manufacturer’s instruction.
2.6 Flow Cytometry
Lymph nodes were teased into a single cell suspension and washed. Red blood cells were lysed with ACK lysing buffer (Cambrex Bio Science, Walkersville, MD) and the remaining lymphocytes were washed again. Cells were incubated with anti-CD3 (145-2C11) and anti-CD19 (1D3) antibodies from Becton, Dickinson and Company for 30 minutes at 4°C, washed, and fixed in 2% paraformaldehyde until analysis. Samples were acquired on a BD FacsCalibur flow cytometer and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
2.7 ELISA
TNF-α levels in BALF were measured using Becton Dickinson’s OptiEIA ELISA kit according to manufacturer’s instructions. Samples from at least six mice were analyzed for each group. For measurement of the anti-F1 antibody response, 96 well plates were coated overnight with 100µl F1 protein at a concentration of 10µg/ml. The plates were then washed 3x, blocked overnight with 250µl of 10% FBS, and then washed again. Samples were added to the wells and incubated overnight at 4°C before washing five times. Horse radish peroxidase conjugated anti-mouse IgG (Jackson ImmunoResearch) or IgA (Southern Biotech) was added to the wells and developed with tetramethlybenzidine (Sigma). After 30 min, development was stopped with 2N H2SO4 and absorbance at 450nm was measured. For all antibody experiments, ten mice were used per group. All samples were analyzed in triplicate.
ELISA to compare antibody avidity were conducted as described above with the addition of a 15 minute incubation of sodium thiocyanate (NaSCN) solution followed by 3 washes prior to incubation with horse radish peroxidase conjugated anti-mouse IgG. The avidity comparison is based on the concentration of NaSCN required to cause a fifty percent reduction in absorbance (Macdonald et al., 1988). In preliminary experiments we tested antibody dilutions ranging from 1:103 to 1:105 and determined 1:104 to be the optimum sample dilution for comparison of avidity.
2.8 Histology and Immunofluorescence
Lungs were perfused for 15 min with 10% formalin following the same procedure used for collection of BAL before removal and transfer to 10% formalin for 24h. The tissue then was trimmed, embedded in paraffin, cut at 4µm, and stained with hematoxylin and eosin by routine methods. The nasal tissue was prepared by removing the skin from the head prior to preserving in 10% formalin overnight, followed by decalcification for 6.5h in Surgipath Decalcifier II solution. A coronal section immediately rostral to the eyes was embedded in paraffin, cut at 4µm and stained with hematoxylin and eosin by routine methods. For histological examination of the lungs and NALT, groups of four mice were used for each condition.
For immunofluorescence lymph nodes were harvested from mice and snap frozen in Optimal Cutting Temperature Compound (OCT) from Sakura (Torrance, CA). Six µm-thick frozen sections were cut from the blocks and dehydrated in acetone. Slides were then blocked with 2% BSA in PBS for 30 min at room temperature. For detection of germinal centers, slides were incubated with the rat anti-mouse IgD antibody 11–26c (eBioscience). After three rinses with 2% BSA in PBS, slides were incubated with AlexaFluor647 labeled goat anti-rat IgG and AlexaFluor568 conjugated peanut agglutinin (Invitrogen). The slides were again washed 3x with 2% BSA in PBS, and coverslips were applied using Prolong Gold antic-fade mounting reagent (Invitrogen). Slides were imaged using a Nikon Eclipse TE300 inverted microscope and a Retiga EX camera. Overlays were composed using Adobe PhotoShop 7.0.
Germinal centers were identified as areas of lymphoid tissue which bound peanut agglutinin (Rose et al., 1980). Tissue sections were cut to a thickness of 6 µm, and every twelfth section throughout the depth of the organ was collected for immunofluorescent analysis. The number of sections analyzed varied depending on the size of each individual lymph node. Generally, between 8 and 12 sections were analyzed per node. Germinal center area was measured in pixels using ImageJ 1.36b (NIH) and then converted to square millimeters. Germinal center volume was calculated by multiplying the average cross sectional area of each germinal center by the depth of the germinal center. The depth of each germinal center was determined by multiplying the number of tissue sections in which the germinal center was detected by 72, which was the number of microns between analyzed sections. Germinal centers which appeared in only one section were assigned a depth of 72 µm.
2.10 Controls & Statistics
The non-signaling mutant flagellin, termed 229, was used as a control in all flagellin experiments. This protein consists of the hypervariable region of flagellin and does not bind TLR5. Recombinant 229 was produced using the same procedure as used for flagellin.
Statistical analysis of data was performed with SigmaStat 3.10 (Systat Software, Inc., Point Richmond, CA). For normally distributed data sets, significance was determined using the Student’s T test. The significance of data sets which were not normally distributed or of unequal variance was determined using the Mann-Whitney rank sum test. P values of less than 0.05 were considered significant. Error bars represent the standard error of the mean.
3. Results
3.1 Flagellin triggers in vivo TNF-α production in aged mice
Toll-like receptor expression in cells from aged mice has been reported to be lower than in cells from young mice (Renshaw et al., 2002), and stimulation of aged macrophages with TLR ligands results in significantly lower levels of TNF-α production than stimulation of young macrophages (Renshaw et al., 2002; Boehmer et al., 2004). To evaluate the ability of flagellin to trigger TNF-α production in vivo in aged mice, we administered flagellin by i.t. instillation and collected BALF 4h later. Instillation of aged mice with flagellin resulted in TNF-α levels in BALF that peaked at 4h and returned to near baseline levels 24h after treatment. The difference in TNF-α levels in BALF from young and aged mice at 4h was not statistically significant (Fig 1A). This result conforms closely to previous findings in young mice (Honko and Mizel, 2004) and demonstrates that aged BALB/c mice are capable of mounting an initial flagellin induced innate immune response similar in magnitude to that of young mice.
Fig. 1.
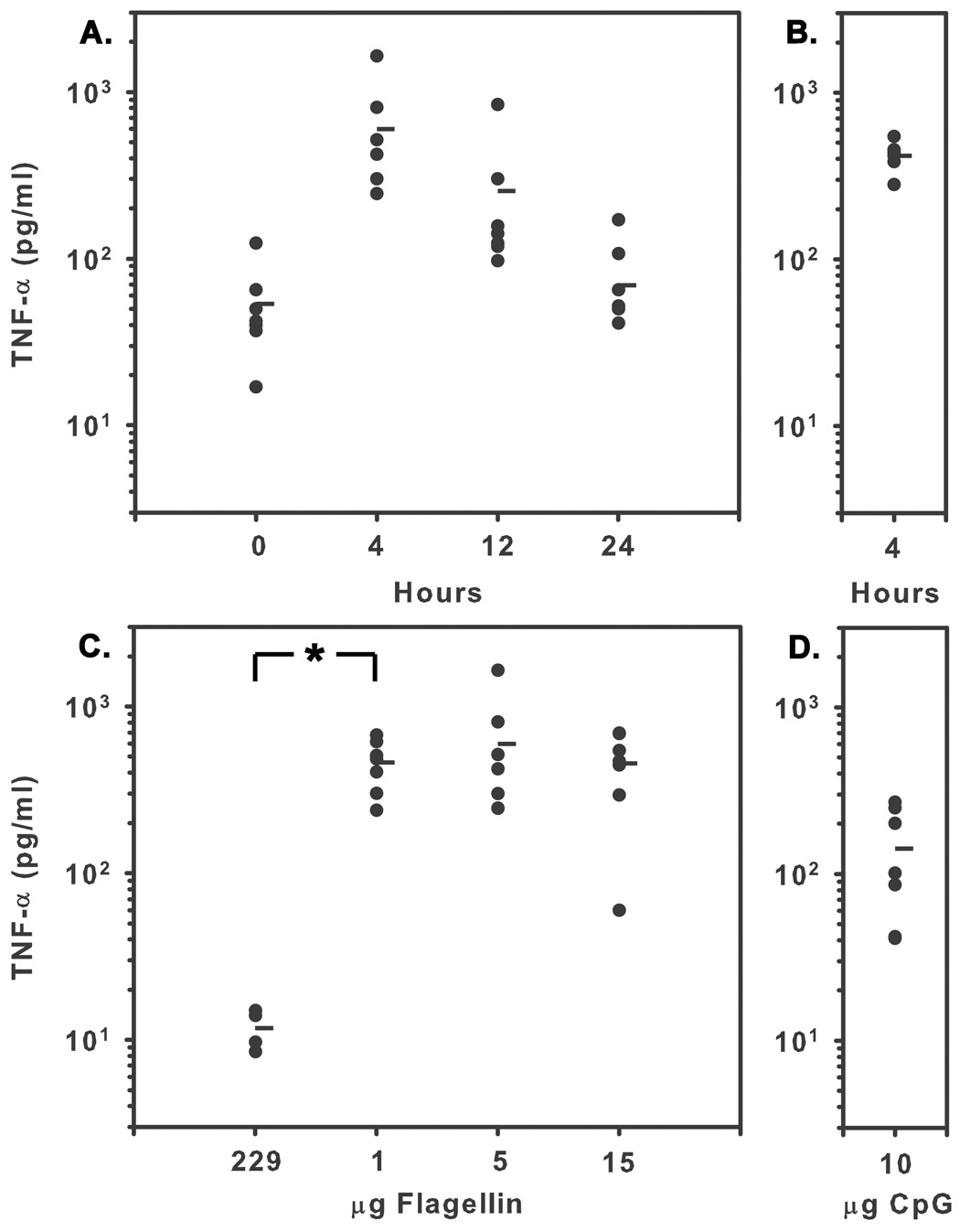
TNF-α levels in BALF from aged mice following intratracheal instillation of flagellin. (A) Aged mice were i.t. instilled with 5 µg of flagellin and sacrificed 4, 12, or 24 hours following treatment or at 0 hours without treatment. (B) Young mice were sacrificed four hours after receiving 5 µg of flagellin. TNF-α levels in aged mice peaked 4 hours after treatment and were not significantly different from levels in young mice at that time (t test, P=0.413). N≥6. (C) Aged mice were i.t. instilled with 5µg of the non-signaling flagellin mutant 229, 1, 5, or 15 µg flagellin. Instillation of 1 µg of flagellin resulted in significantly higher TNF-α levels than 229 (Mann-Whitney rank sum test, *P=0.006) or CpG (t test, P<0.001), however, instillation of 1 to 15 µg of flagellin did not result in significantly different levels of TNF-α (t test, P=0.961). (D) I.t. Instillation of 10 µg CpG resulted in lower TNF-α levels than 1µg of flagellin. Horizantal bars represent the mean.
In vivo studies examining cytokine production and lethality in aged mice following challenge with LPS have shown that aged mice are more susceptible to LPS challenge than young mice (Tateda et al., 1996). While these studies involved treatment of mice with extraordinarily high doses of LPS, they do raise the possibility that aged mice may exhibit an altered dose response to lower doses of TLR ligands. Previously published studies have shown that instillation of 5 µg of flagellin is required to achieve maximal TNF-α levels in the lungs of young mice (Honko and Mizel, 2004). We evaluated the dose response to flagellin in aged mice by i.t. instilling 5 µg 229 control protein, 1, 5, 15 µg flagellin, or 10µg CpGODN as a positive control and measuring TNF-α levels in BALF 4h later. Maximal TNF-α levels were achieved with of 1 µg of flagellin (Fig 1B). These levels were not significantly different from TNF-α levels following instillation of 5 or 15 µg of flagellin (P>0.05). Notably, the level of TNF-α present following instillation of 10 µg CpG-ODN was markedly less than the level achieved following instillation of 1 to 15 µg of flagellin (Fig 1B). Intratracheal instillation of flagellin in aged mice did not result in detectable levels of TNF-α in the plasma (data not shown). These results are consistent with the hypothesis that flagellin triggers an innate immune response in aged mice and that aged mice are more sensitive to flagellin than younger mice.
3.2 Flagellin triggers an acute innate response in the lungs of aged mice
To further characterize the innate immune response to flagellin in aged mice, 5 µg of flagellin or 229 control protein was instilled i.t., and 12h later lung tissues were collected for histological evaluation. Lungs from flagellin-treated aged mice exhibited acute inflammation. Diffuse hyperemia and peribronchial neutrophil infiltrates were present to similar degrees in both age groups, while controls were within normal limits (Fig. 2). Bronchial associated lymphoid tissue was present in both flagellin treated and 229 control aged mice. Comparison of tissue sections from untreated aged and young mice revealed that aged mice consistently have more bronchus associated lymphoid tissue than young mice (N=3, data not shown).
Fig. 2.
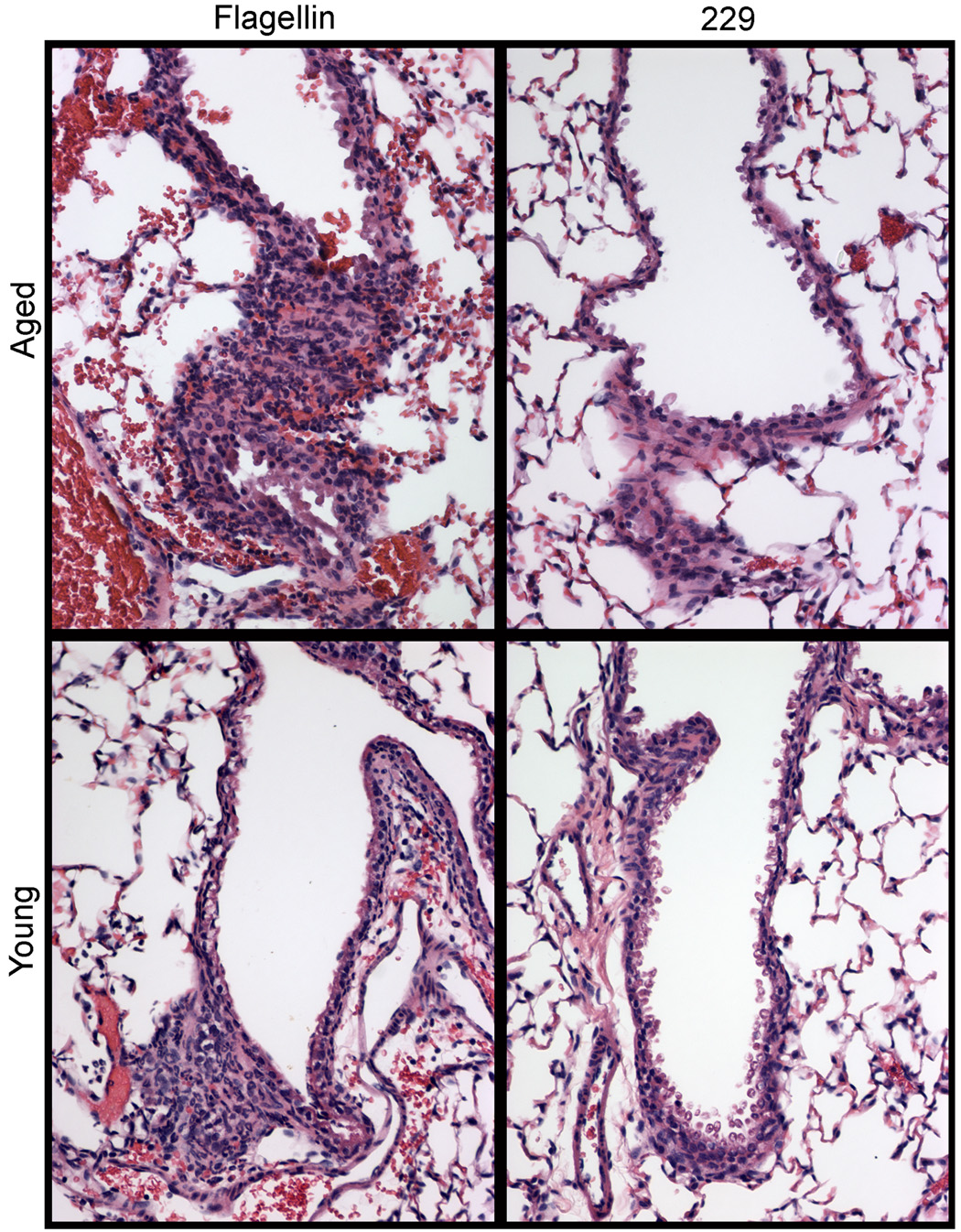
Innate response in the lungs of aged and young mice sacrificed 12 hours after i.t. instillation with 5 µg of flagellin or the control protein 229. The innate response in both age groups was characterized by diffuse hyperemia and peribronchial neutrophil infiltrates. Tissues from control mice were unaffected.
Though acute inflammation in the lungs of young and aged mice following instillation of flagellin appeared similar, we also evaluated the numbers and types of cells recovered from BALF. To quantify the cellular infiltrate into the lungs of aged and young mice, we i.t. instilled aged mice with 5 µg of flagellin, and sacrificed mice at 4, 12, and 24h following treatment. Differential cell counts were performed on cell populations recovered from bronchoalveolar lavage fluid. Young mice were also instilled with 5 µg flagellin and sacrificed at 12h. As demonstrated for young mice (Honko and Mizel, 2004), inflammatory cell infiltration in the lungs of aged mice became maximal 12h after flagellin instillation and was largely resolved by 24h. However, slightly more than 3 times as many cells were recovered from the BALF of aged mice at the peak of the response as from young mice in the present study (Fig. 3A). Consistent with lung histology, neutrophils were overwhelmingly the dominant infiltrating cell type in both young and aged mice (Fig 3B).
Fig. 3.
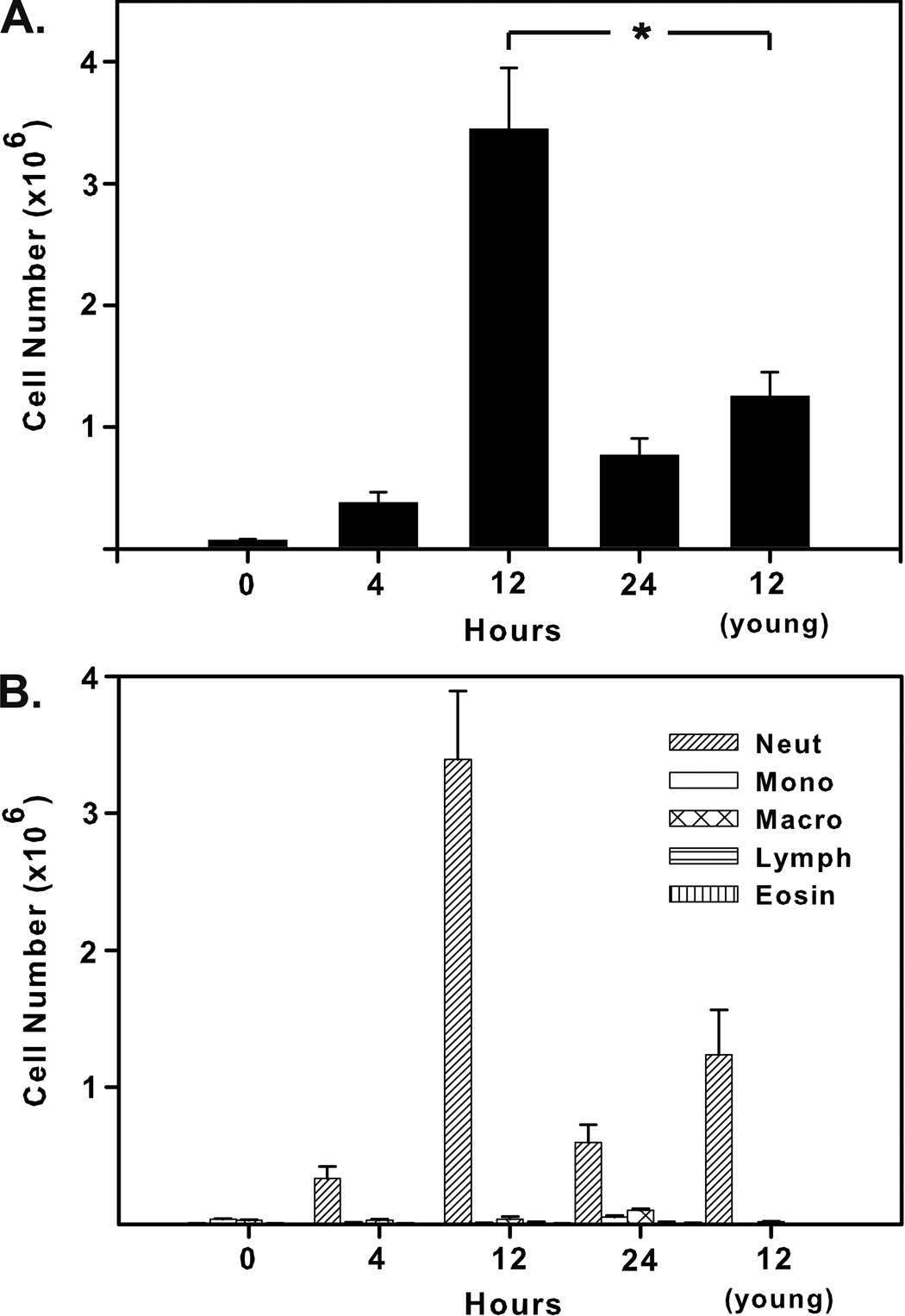
Cellular infiltrate in the lungs of aged mice following i.t. instillation of flagellin. Aged mice were instilled with 5 µg of flagellin and sacrificed 4, 12, or 24 hours following treatment or at 0 hours without treatment. Young mice were sacrificed 12 hours after receiving flagellin. (A) Total cellular infiltrate peaked in aged mice 12 hours after treatment and was 2.8 times greater than levels in young mice at that time point (t test, *P=0.002). (B) Neutrophils were the dominant infiltrating cell type in young and aged mice at all observed times.
3.3 Flagellin triggers cellular infiltrate in the upper respiratory tract of aged mice
Previous work has shown that i.n. immunization of young mice with flagellin is highly effective at promoting protective immunity (McEwen et al., 1992; Levi and Arnon, 1996; Ben-Yedidia et al., 1999; Honko et al., 2006). To compare the innate response to i.n. administered flagellin in aged and young mice, we instilled mice with 5 µg of flagellin or the control protein 229 and sacrificed mice 12h following treatment. Histological evaluation demonstrated similar tissue changes and acute inflammation in the nasal tissue of young and aged mice 12h after i.n. instillation of flagellin, compared to control animals. For example, neutrophil-rich exudates along the respiratory epithelium in the nasal cavity and loss of cilia, vacuolation and individual cell loss among the respiratory epithelial cells were evident (Fig. 4). None of these changes were observed in the control animals.
Fig. 4.
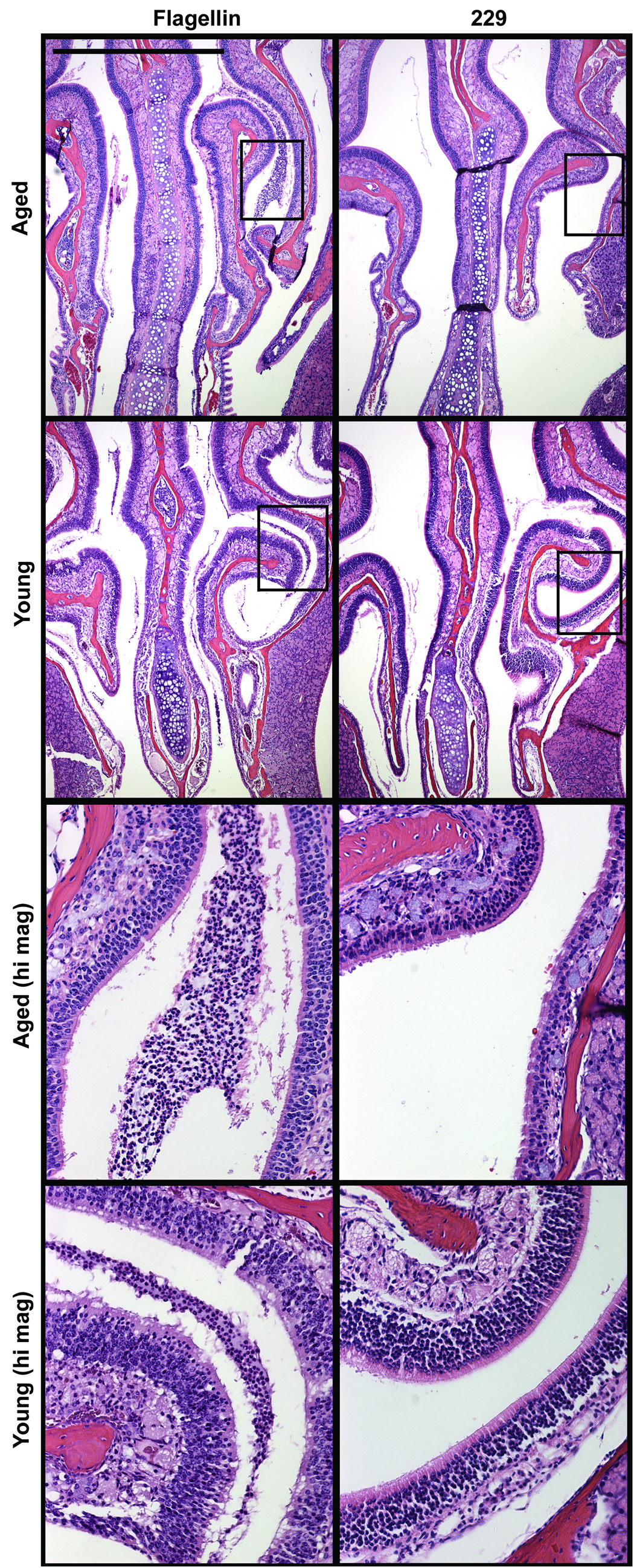
Cellular infiltrate in the nasal passageways of aged and young mice 12 hours following i.n. instillation of 5 µg of flagellin or the control protein 229. Neutrophil-rich exudates collected along the respiratory epithelium of aged and young mice. Loss of cilia, vacuolation, and individual cell loss among the respiratory epithelial cells were similar in aged and young mice. Control mice were unaffected. Scale bar represent 1 mm.
TNF-α was undetectable in nasal wash fluids or BALF 4h following i.n. instillation of flagellin (data not shown). Histological evaluation of lungs from mice 12h following treatment with flagellin was essentially normal, although slightly more cells were recovered in BALF of flagellin treated mice compared to controls (data not shown). Given the importance of the innate response in the development of an effective adaptive response (Pulendran and Ahmed, 2006), flagellin’s ability to trigger a robust but transient innate response in the respiratory compartment of aged mice is highly significant.
3.4 Flagellin results in increased cellularity in draining lymph nodes
To test the hypothesis that flagellin promotes T and B lymphocyte recruitment into draining lymph nodes, we i.n. instilled groups of 5 young and aged BALB/c mice with 5 µg of flagellin or the control protein 229. Twenty-four hours following treatment, the numbers of total cells, T cells, and B cells in the cranial deep cervical, mandibular, and superficial parotid lymph nodes were determined (Fig. 5). (See (Van den Broeck et al., 2006) for a detailed description and standardized nomenclature of murine lymph nodes.) Instillation of flagellin had the greatest effect in the cranial deep cervical lymph nodes where instillation caused a 2.7-fold increase in total cell number in young mice (Fig 5A) and a 3.6-fold increase in aged mice (Fig 5B). Increases in the numbers of T and B cells accounted for nearly all of the increased cellularity in these nodes. Additionally, T and B cells were recruited at similar levels, thus treatment with flagellin does not preferentially recruit T rather than B cells to the draining lymph nodes. Flagellin-treated mice also had increased cell numbers in the mandibular lymph nodes in both age groups, though this increase was not statistically significant (P>0.07). In contrast, the numbers of cells in the superficial parotid lymph nodes of young mice decreased somewhat following i.n. administration of flagellin, though treatment had no effect on numbers of cells present in these lymph nodes in aged mice (Fig 5C). The increases in cell numbers at this short time point after instillation of flagellin likely reflect a generic chemokine-mediated recruitment of lymphocytes from the blood rather than an increase in the number of flagellin-specific lymphocytes. These changes provide a mechanism that increases the likelihood of lymphocytes encountering their cognate antigen in an environment supportive of a productive immune response. Treatment of mice with flagellin did not result in increased CD69 expression by T cells in draining lymph nodes (data not shown).
Fig. 5.
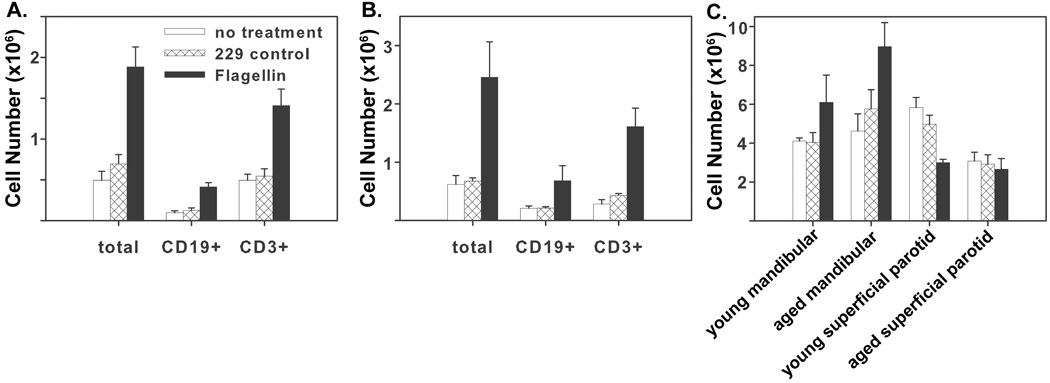
Increased cellularity in the cranial deep cervical lymph nodes of young and aged mice following i.n. administration of flagellin. Young and aged mice were i.n. instilled with 5 µg of flagellin and sacrificed 24 hours later. The number of total cells, CD19+, and CD3+ cells in the cranial deep cervical lymph nodes increased in (A) young and (B) aged mice following treatment with flagellin compared to 229 (P<0.02). (C) Increases in cell number in mandibular lymph nodes of young and aged mice following treatment with flagellin were not statistically significantly (P>0.07), and no increase in cell number occurred in the superficial parotid lymph nodes.
3.5 Flagellin promotes antigen-specific antibody production in aged and young mice
In view of our finding that flagellin promoted recruitment or retention of large numbers of T and B cells into the draining lymph nodes of aged mice, we hypothesized that immunization with flagellin would also be effective at promoting antigen-specific antibody formation in aged mice. Groups of 10 mice were i.n. immunized with 1 µg of flagellin or the control protein 229 and 10 µg of the Y. pestis protein F1. Four weeks following primary immunization, mice were boosted with a repeat dose of the primary immunization, and sacrificed 7 days later. In aged mice, immunization with flagellin and F1 resulted in anti-F1 IgA titers of 38 in nasal wash and 13 in BALF. Young mice had anti-F1 IgA titers of 108 in nasal wash fluid and 56 in BALF. The difference in nasal wash anti-F1 IgA titers between the two age groups was not statistically significant (P=0.054). Immunization with the control protein 229 and F1 protein did not result in detectable IgA titers in either age group (Fig 6A). Immunization with flagellin and F1 also induced anti-F1 IgG titers in nasal wash fluid, BALF, and plasma of young and aged mice. Aged mice had anti-F1 IgG titers of 48 in nasal wash, 805 in BALF, and 4.1×105 in plasma. As with anti-F1 IgA, the response was somewhat less compared to that in young mice which had anti-F1 IgG titers of 530 in nasal wash, 4800 in BALF, and 2.8×106 in plasma. IgG levels in mice immunized with the control protein 229 and F1 protein were below the limit of detection in both mucosal compartments and less than 1000 in plasma (Fig 6B). These results clearly establish that flagellin is an effective adjuvant, promoting systemic and mucosal immune responses, in aged mice though not to levels as high as in young mice.
Fig. 6.
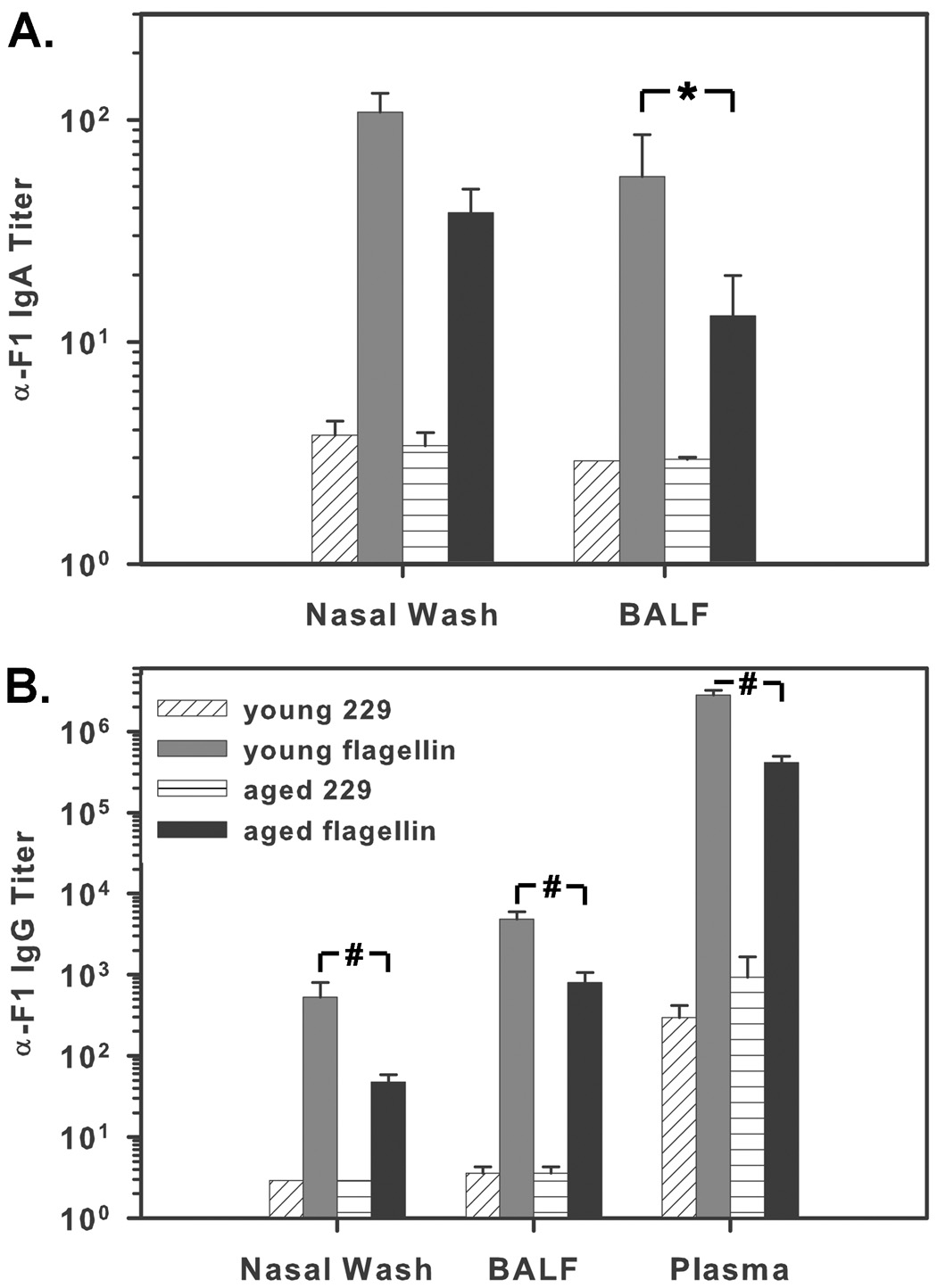
Antigen-specific antibody responses in young and aged mice. Mice were i.n. immunized with 1 µg of flagellin or the control protein 229 and 10 µg of F1. Mice were boosted on day 28 and sacrificed 7 days following boost. (A) Anti-F1 IgA titers were measured in nasal wash and BALF. Differences in NW IgA levels in flagellin-immunized young and aged mice were not statistically significant (P=0.054), though differences in BALF IgA levels were significant (*P=0.007). (B) Anti-F1 IgG titers were determined in nasal wash fluid, BALF, and plasma. Immunization with flagellin promotes F1-specific antibody formation in aged mice; however, IgG levels in flagellin-immunized aged mice are significantly lower than in flagellin-immunized young mice in all observed compartments (#P≤0.001).
3.6 Flagellin promotes germinal center formation in young but not aged mice
Given the lower titer of F1-specific antibodies in aged mice, we considered the possibility that antigen-specific B cell expansion might be more limited in aged mice. To evaluate the ability of flagellin to promote germinal center formation in aged and young mice, we i.n. immunized groups of 5 to 6 mice with 1 µg of flagellin or the control protein 229 and 10 µg of the Y. pestis protein F1 and sacrificed mice ten days later. Germinal centers in the cranial deep cervical lymph nodes were discriminated on the basis PNA binding and IgD downregulation (Fig 7A). Immunization of young mice with flagellin and F1 protein resulted in significantly greater numbers of germinal centers per lymph node than in control mice (Fig 7B). These germinal centers were also significantly greater in volume. The numbers of germinal centers in lymph nodes from aged mice were highly variable in both flagellin and 229 immunized groups. Immunization of aged mice with flagellin did not produce a significant difference in number or volume of germinal centers compared to aged 229 immunized control mice. The lack of an effect of flagellin on germinal center formation in aged mice may be responsible in part for the reduced F1-specific antibody titer in aged mice.
Fig. 7.
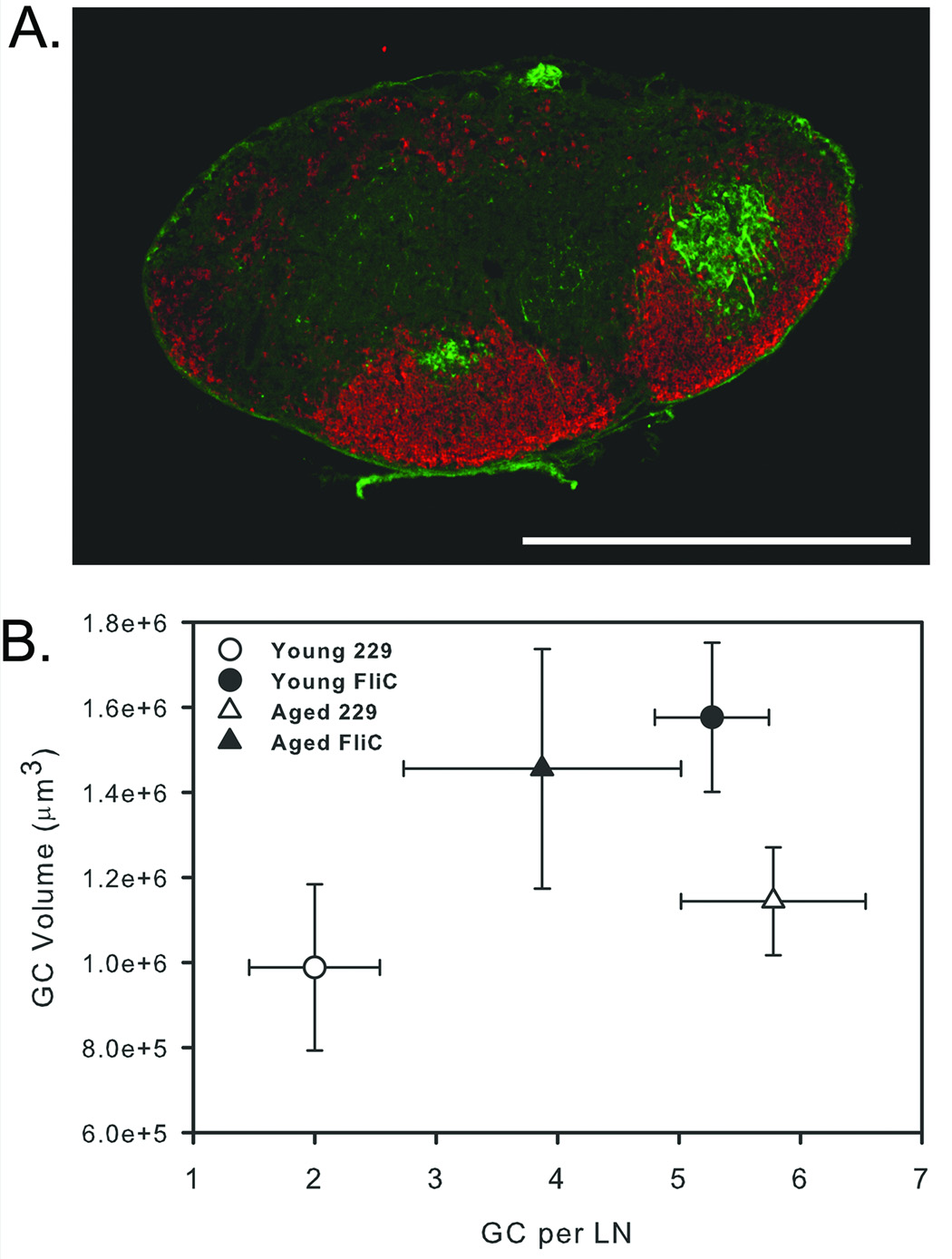
Number and volume of germinal centers in aged and young mice following immunization. Mice were i.n. instilled with 1 µg of flagellin or 229 control protein and 10 µg of F1. Mice were sacrificed 10 days later, and the number and volume of germinal centers in cranial deep cervical lymph nodes was analyzed by immunofluorescence. (A) Germinal centers were detected based on peanut agglutinin (green) binding ability, and B cell follicles were identified based on IgD expression (red). (B) Immunization of young mice with flagellin resulted in significantly larger and more germinal centers than with 229 (P<0.05). Germinal center number and volume was highly variable in aged mice and differences in number and volume of germinal centers in flagellin and 229 control immunized mice was not significant (P>0.05). Scale bar represents 1 mm.
3.7 Reduced Antibody Avidity in Aged Mice
In view of the more limited germinal center formation in aged mice following immunization, we considered the possibility that antibodies from aged mice may be of lower avidity than antibodies from young mice. Plasma samples from groups of 10 young and aged BALB/c mice immunized with 1 µg of flagellin or 229 and 10 µg of F1 were analyzed by ELISA. Avidity was compared on the basis of antigen-bound antibodies’ resistance to elution by sodium thiocyanate. The avidity index represents the concentration of sodium thiocyanate required to reduce absorbance as measured by ELISA by 50%. The average avidity index of plasma samples from young mice was 3M NaSCN while samples from aged mice had a significantly lower (P=0.002) average avidity index of 1M NaSCN (Fig 8A). Samples from aged mice showed more heterogeneity in their sensitivity to NaSCN than samples from young mice (Fig 8B & 8C). Incubation in 0.1 M NaSCN was sufficient to cause elution of some aged samples, while samples from young mice were unaffected by incubation with 1M NaSCN. These results establish that on average, antibodies from aged mice are less avid than antibodies from young mice. However, some aged mice are still capable of producing antibodies with avidities equal to antibodies from young mice.
Fig. 8.
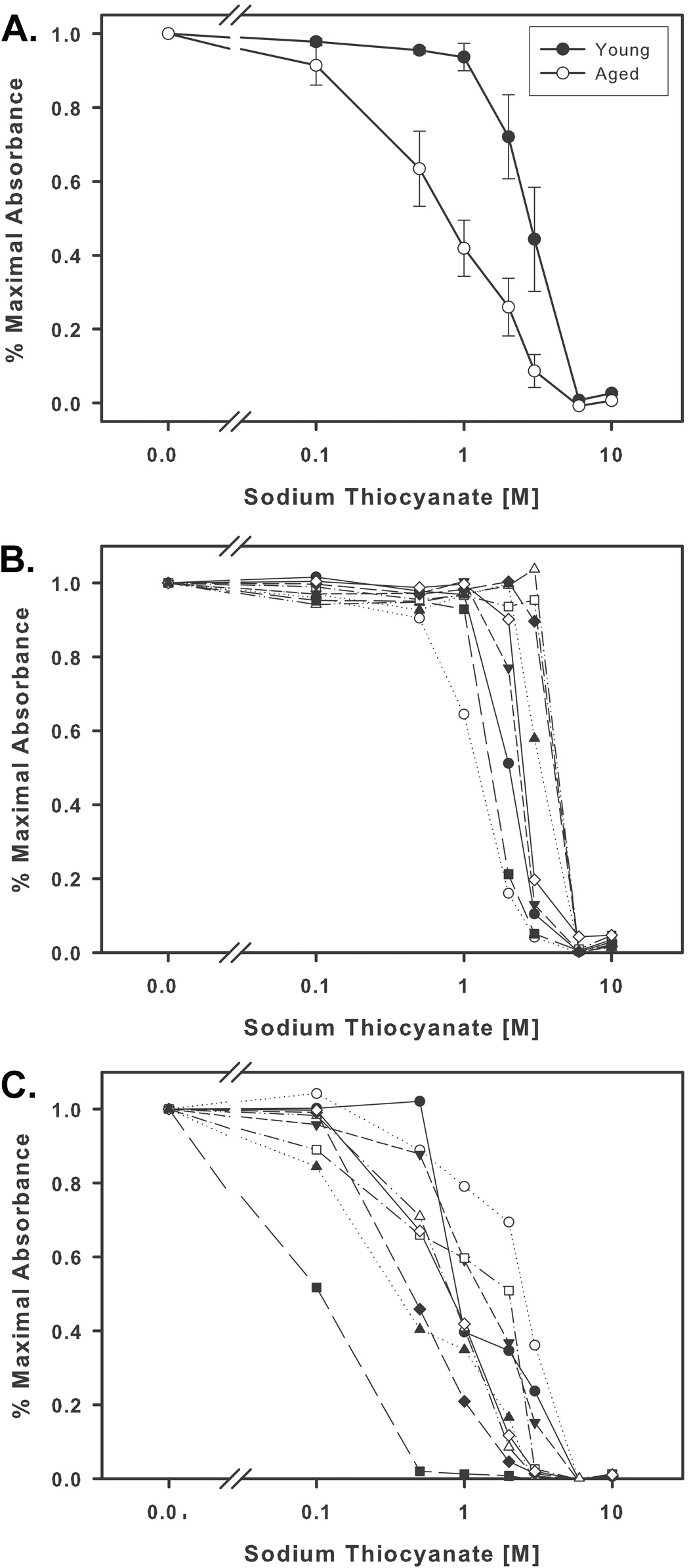
Avidity of anti-F1 plasma IgG in immunized young and aged mice. (A) Antibodies from aged mice exhibit lower avidity than antibodies from young mice. Incubation in approximately 1 M NaSCN was sufficient to reduce absorbance of aged plasma IgG by 50%, but young plasma IgG did not exhibit 50% reduction in absorbance until incubation with 3 M NaSCN (N=9, P=0.002). Variation of the concentration of NaSCN necessary to result in 50% reduction in absorbance was less in young mice (B) than in aged mice (C). Calculation of the standard deviation of the 50% reduction concentrations for young and aged mice yielded a standard deviation of 0.20 logarithmic units for the samples from young mice and 0.41 for the samples from aged mice.
4. Discussion
We have shown that mucosal administration of purified, endotoxin-depleted recombinant flagellin promotes a potent, but transient respiratory innate immune response in aged mice. As a mucosal adjuvant, flagellin also promoted a significant adaptive immune response in aged mice. In a related study (Ben-Yedidia et al., 1998), Ben-Yedidia and colleagues reported that intranasal immunization with flagellin-HA and flagellin-NP fusion proteins resulted in immunity to influenza in aged mice but that aged BALB/c mice had lower HA specific IgG and IgA titers following immunization than 3 month old mice.
The most notable finding in our study was that although flagellin evokes a stronger innate immune response in aged mice than in young mice, the increased innate response in aged mice fails to consistently promote a correspondingly strong adaptive response. Several other groups have reported increased response to TLR stimulation (Habicht, 1981; Esposito et al., 1989; Chorinchath et al., 1996; Gomez et al., 2007) or infection (Esposito et al., 1990) in aged mice. Gomez and colleagues reported that instillation of LPS in aged mice triggered increased levels of MIP-2, KC, and IL-1β in lung homogenates as well as increased infiltration of neutrophils compared to young mice (Gomez et al., 2007). However, TNF-α levels were not significantly different in young and aged mice. The reasons underlying the increased innate response in aged mice are unclear. Aoshiba and Nagai performed microarray analysis on cDNA from lungs of young and aged mice and found that several inflammation related genes, including IL-8RB and CXCR3 are more highly expressed in aged mice than in young mice (Aoshiba and Nagai, 2007). One likely explanation for these age-related differences is a diminished ability by aged mice to negatively regulate innate stimulatory pathways. Several lines of evidence have implicated phosphoinosotide-3-kinase (PI3K) in the altered regulation of innate immunity. For example, Ong and colleagues have shown that neutrophil recruitment into the lungs of PI3K gamma deficient mice is increased 100% compared to wild type mice in an E. coli sepsis model system (Ong et al., 2005). In another sepsis model system, inhibition of PI3K resulted in increased cytokine production as well as decreased survival time of septic mice (Williams et al., 2004). Importantly, inhibition of PI3K prior to stimulation of epithelial cells with flagellin resulted in increased expression of nitric oxide synthase, IL-6, and IL-8 (Yu et al., 2006). Thus our data showing increased neutrophil infiltration into the lungs of aged mice after treatment with flagellin compared to young mice are consistent with the hypothesis that aged mice have a reduced capacity to signal through PI3K that, in turn, results in an increased innate immune responsiveness to flagellin.
In addition to negatively regulating components of the innate immune response, the PI3K pathway upregulates phagocytosis (Cox et al., 2001), is necessary for DC migration in vivo (Del Prete et al., 2004), and may also negatively regulate TLR signaling (Guha and Mackman, 2002; Wrann et al., 2007). Agrawal and colleagues have reported that DC from aged humans exhibit a reduced ability to phagocytize antigens and migrate in response to chemokines. They also found that DC from aged humans produce higher levels of TNF-α and IL-6 in response to stimulation with LPS and ssRNA compared to DC from young humans (Agrawal et al., 2007). They attributed these changes in aged DC to reduced signaling through the PI3K pathway as measured by phosphorylation of AKT and found that expression of PTEN, a negative regulator of PI3K (Cantley and Neel, 1999), was increased in DC from aged humans. PTEN has also been shown to negatively regulate the CD4+ T cell (Suzuki et al., 2001) and B cell (Anzelon et al., 2003; Suzuki et al., 2003) responses. Thus decreased PI3K signaling resulting from increased PTEN expression in aged mice may account for the diminished adaptive response to immunization as well as the increased innate response. If reduced flux through the PI3K signaling pathway is responsible for the diminished adaptive response in aged mice, then it may be possible to enhance these responses by additional immunizations with flagellin and antigen or by pharmacological modulation of PTEN activity at the time of immunization.
Due to the recent National Institute of Aging mandated change in the availability of aged mice, we are currently unable to test the efficacy of our vaccine in aged mice or to proceed with studies on the optimization of the vaccination protocol (e.g., additional immunizations, increased dose, or alternate route of administration). Given the susceptibility of aged populations to respiratory pathogens and the reduced efficacy of vaccines among the elderly, our demonstration that flagellin promotes significant mucosal and systemic adaptive immune responses in aged mice is of major importance and thus clearly merits additional study. We believe that flagellin has significant potential for use as a vaccine adjuvant in humans. Indeed, we are very close to completing preclinical development of a flagellin/F1/V fusion protein for use human clinical trials. The flagellin/F1/V fusion protein vaccine has been produced and vialed under Good Manufacturing Practices conditions and has passed all of its quality control tests.
Acknowledgments
We gratefully acknowledge Tomasz Szul for helpful suggestions.
This work was supported by a grant from the National Institutes of Health, P01 AI 60642 (to S.B.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Agrawal S, Cao JN, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- Alignani D, Maletto B, Liscovsky M, Ropolo A, Moron G, Pistoresi-Palencia MC. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J Leukoc Biol. 2005;77:898–905. doi: 10.1189/jlb.0604330. [DOI] [PubMed] [Google Scholar]
- Anzelon AN, Wu H, Rickert RC. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol. 2003;4:287–294. doi: 10.1038/ni892. [DOI] [PubMed] [Google Scholar]
- Aoshiba K, Nagai A. Chronic lung inflammation in aging mice. FEBS Lett. 2007;581:3512–3516. doi: 10.1016/j.febslet.2007.06.075. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Hirokawa K, Uchiyama M, Suzuki Y, Aizawa C, Kurata T, Sata T, Tamura S. Immune responses and protection in different strains of aged mice immunized intranasally with an adjuvant-combined influenza vaccine. Vaccine. 2001;19:3981–3989. doi: 10.1016/s0264-410x(01)00129-3. [DOI] [PubMed] [Google Scholar]
- Ben-Yedidia T, Abel L, Arnon R, Globerson A. Efficacy of anti-influenza peptide vaccine in aged mice. Mech Ageing Dev. 1998;104:11–23. doi: 10.1016/s0047-6374(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Ben-Yedidia T, Tarrab-Hazdai R, Schechtman D, Arnon R. Intranasal administration of synthetic recombinant peptide-based vaccine protects mice from infection by Schistosoma mansoni. Infect Immun. 1999;67:4360–4366. doi: 10.1128/iai.67.9.4360-4366.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorinchath BB, Kong LY, Mao L, McCallum RE. Age-associated differences in TNF-alpha and nitric oxide production in endotoxic mice. J Immunol. 1996;156:1525–1530. [PubMed] [Google Scholar]
- Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18) J Exp Med. 2001;193:61–71. doi: 10.1084/jem.193.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadros C, Lopez-Hernandez FJ, Dominguez AL, McClelland M, Lustgarten J. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect Immun. 2004;72:2810–2816. doi: 10.1128/IAI.72.5.2810-2816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey RW, Eun SY, Russell CE, Vogel LA. B cells of aged mice show decreased expansion in response to antigen, but are normal in effector function. Cell Immunol. 2001;214:99–109. doi: 10.1006/cimm.2001.1894. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, Facchetti F, Wymann MP, Vecchi A, Hirsch E, Mantovani A, Sozzani S. Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. Embo J. 2004;23:3505–3515. doi: 10.1038/sj.emboj.7600361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- Dennis C. Flu-vaccine makers toil to boost supply. Nature. 2006;440:1099. doi: 10.1038/4401099a. [DOI] [PubMed] [Google Scholar]
- Ehrchen J, Sindrilaru A, Grabbe S, Schonlau F, Schlesiger C, Sorg C, Scharffetter-Kochanek K, Sunderkotter C. Senescent BALB/c mice are able to develop resistance to Leishmania major infection. Infect Immun. 2004;72:5106–5114. doi: 10.1128/IAI.72.9.5106-5114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shaikh KA, Gabry MS, Othman GA. Recovery of age-dependent immunological deterioration in old mice by thyroxine treatment. J Anim Physiol Anim Nutr (Berl) 2006;90:244–254. doi: 10.1111/j.1439-0396.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- Esposito AL, Poirier WJ, Clark CA. In vitro assessment of chemotaxis by peripheral blood neutrophils from adult and senescent C57BL/6 mice: correlation with in vivo responses to pulmonary infection with type 3 Streptococcus pneumoniae. Gerontology. 1990;36:2–11. doi: 10.1159/000213169. [DOI] [PubMed] [Google Scholar]
- Esposito AL, Poirier WJ, Clark CA, Brown ML. The release of neutrophil chemoattractant activity by bronchoalveolar macrophages from adult and senescent mice. J Gerontol. 1989;44:B93–B99. doi: 10.1093/geronj/44.4.b93. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Miller RA. Age-dependent defects in TCR-triggered cytoskeletal rearrangement in CD4+ T cells. J Immunol. 2002;169:5021–5027. doi: 10.4049/jimmunol.169.9.5021. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Hirano S, Cutro BT, Birjandi S, Baila H, Nomellini V, Kovacs EJ. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: A quantitative review. Vaccine. 2006;24:1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Habicht GS. Body temperature in normal and endotoxin-treated mice of different ages. Mech Ageing Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honko AN, Mizel SB. Mucosal administration of flagellin induces innate immunity in the mouse lung. Infect Immun. 2004;72:6676–6679. doi: 10.1128/IAI.72.11.6676-6679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honko AN, Sriranganathan N, Lees CJ, Mizel SB. Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun. 2006;74:1113–1120. doi: 10.1128/IAI.74.2.1113-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Rozzo SJ, Cambier JC. Aging-dependent exclusion of antigen-inexperienced cells from the peripheral B cell repertoire. J Immunol. 2002;168:5014–5023. doi: 10.4049/jimmunol.168.10.5014. [DOI] [PubMed] [Google Scholar]
- Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–681. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- Keitel WA, Atmar RL, Cate TR, Petersen NJ, Greenberg SB, Ruben F, Couch RB. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006;166:1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- Kolibab K, Smithson SL, Rabquer B, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable heavy chain repertoire. Infect Immun. 2005;73:7465–7476. doi: 10.1128/IAI.73.11.7465-7476.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck- Loebenstein B. Age-related loss of naive T cells and dysregulation of T-cell/B-cell interactions in human lymph nodes. Immunology. 2005;114:37–43. doi: 10.1111/j.1365-2567.2004.02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi R, Arnon R. Synthetic recombinant influenza vaccine induces efficient long-term immunity and cross-strain protection. Vaccine. 1996;14:85–92. doi: 10.1016/0264-410x(95)00088-i. [DOI] [PubMed] [Google Scholar]
- Lewis DJ, Eiden JE, Goilav C, Langenberg AG, Suggett F, Griffin GE. Rapid and frequent induction of protective immunity exceeding UK recommendations for healthcare settings by MF59-adjuvated hepatitis B vaccine. Commun Dis Public Health. 2003;6:320–324. [PubMed] [Google Scholar]
- Lowy J, Hanson J. Structure of Bacterial Flagella. Nature. 1964;202:538–540. doi: 10.1038/202538a0. [DOI] [PubMed] [Google Scholar]
- Lowy J, McDonough MW. Structure of Filaments Produced by Re-Aggregation of Salmonella Flagellin. Nature. 1964;204:125–127. doi: 10.1038/204125a0. [DOI] [PubMed] [Google Scholar]
- Macdonald RA, Hosking CS, Jones CL. The measurement of relative antibody affinity by ELISA using thiocyanate elution. J Immunol Methods. 1988;106:191–194. doi: 10.1016/0022-1759(88)90196-2. [DOI] [PubMed] [Google Scholar]
- McDermott PF, Ciacci-Woolwine F, Snipes JA, Mizel SB. High-affinity interaction between gram-negative flagellin and a cell surface polypeptide results in human monocyte activation. Infect Immun. 2000;68:5525–5529. doi: 10.1128/iai.68.10.5525-5529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen J, Levi R, Horwitz RJ, Arnon R. Synthetic recombinant vaccine expressing influenza haemagglutinin epitope in Salmonella flagellin leads to partial protection in mice. Vaccine. 1992;10:405–411. doi: 10.1016/0264-410x(92)90071-q. [DOI] [PubMed] [Google Scholar]
- McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J Immunol. 2002;169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–5175. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- Miller RA. Defective calcium signal generation in a T cell subset that accumulates in old mice. Ann N Y Acad Sci. 1989;568:271–276. doi: 10.1111/j.1749-6632.1989.tb12516.x. [DOI] [PubMed] [Google Scholar]
- Mittler JN, Lee WT. Antigen-specific CD4 T cell clonal expansion and differentiation in the aged lymphoid microenvironment. I. The primary T cell response is unaffected. Mech Ageing Dev. 2004;125:47–57. doi: 10.1016/j.mad.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Montes CL, Maletto BA, Acosta Rodriguez EV, Gruppi A, Pistoresi-Palencia MC. B cells from aged mice exhibit reduced apoptosis upon B-cell antigen receptor stimulation and differential ability to up-regulate survival signals. Clin Exp Immunol. 2006;143:30–40. doi: 10.1111/j.1365-2249.2005.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Newton SM, Jacob CO, Stocker BA. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989;244:70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Ong E, Gao XP, Predescu D, Broman M, Malik AB. Role of phosphatidylinositol 3-kinase-gamma in mediating lung neutrophil sequestration and vascular injury induced by E. coli sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1094–L1103. doi: 10.1152/ajplung.00179.2005. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Pino O, Martin M, Michalek SM. Cellular Mechanisms of the Adjuvant Activity of the Flagellin Component FljB of Salmonells enterica Serovar Typhimurium To Potentiate Mucosal and Systemic Responses. Infect Immun. 2005;73:6763–6770. doi: 10.1128/IAI.73.10.6763-6770.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167:584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Roos-Van Eijndhoven DG, Cools HJ, Westendorp RG, Ten Cate-Hoek AJ, Knook DL, Remarque EJ. Randomized controlled trial of seroresponses to double dose and booster influenza vaccination in frail elderly subjects. J Med Virol. 2001;63:293–298. doi: 10.1002/1096-9071(200104)63:4<293::aid-jmv1004>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rose ML, Birbeck MS, Wallis VJ, Forrester JA, Davies AJ. Peanut lectin binding properties of germinal centres of mouse lymphoid tissue. Nature. 1980;284:364–366. doi: 10.1038/284364a0. [DOI] [PubMed] [Google Scholar]
- Saurwein-Teissl M, Lung TL, Marx F, Gschosser C, Asch E, Blasko I, Parson W, Bock G, Schonitzer D, Trannoy E, Grubeck-Loebenstein B. Lack of antibody production following immunization in old age: association with CD8(+)CD28(−) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- Sen G, Chen Q, Snapper CM. Immunization of aged mice with a pneumococcal conjugate vaccine combined with an unmethylated CpG-containing oligodeoxynucleotide restores defective immunoglobulin G antipolysaccharide responses and specific CD4+-T-cell priming to young adult levels. Infect Immun. 2006;74:2177–2186. doi: 10.1128/IAI.74.4.2177-2186.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithson SL, Kolibab K, Shriner AK, Srivastava N, Khuder S, Westerink MA. Immune response to pneumococcal polysaccharides 4 and 14 in elderly and young adults: analysis of the variable light chain repertoire. Infect Immun. 2005;73:7477–7484. doi: 10.1128/IAI.73.11.7477-7484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strindelius L, Filler M, Sjoholm I. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine. 2004;22:3797–3808. doi: 10.1016/j.vaccine.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med. 2003;197:657–667. doi: 10.1084/jem.20021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, Tsubata T, Ohashi PS, Koyasu S, Penninger JM, Nakano T, Mak TW. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity. 2001;14:523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–1251. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K. Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun. 1996;64:769–774. doi: 10.1128/iai.64.3.769-774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomer Y, Mendlovic S, Kukulansky T, Mozes E, Shoenfeld Y, Globerson A. Effects of aging on the induction of experimental systemic lupus erythematosus (SLE) in mice. Mech Ageing Dev. 1991;58:233–244. doi: 10.1016/0047-6374(91)90095-h. [DOI] [PubMed] [Google Scholar]
- Troutaud D, Drouet M, Decourt C, Le Morvan C, Cogne M. Age-related alterations of somatic hypermutation and CDR3 lengths in human Vkappa4-expressing B lymphocytes. Immunology. 1999;97:197–203. doi: 10.1046/j.1365-2567.1999.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeck W, Derore A, Simoens P. Anatomy and nomenclature of murine lymph nodes: Descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods. 2006;312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- van Dijk-Hard I, Soderstrom I, Feld S, Holmberg D, Lundkvist I. Age-related impaired affinity maturation and differential D-JH gene usage in human VH6-expressing B lymphocytes from healthy individuals. Eur J Immunol. 1997;27:1381–1386. doi: 10.1002/eji.1830270613. [DOI] [PubMed] [Google Scholar]
- Vannier E, Borggraefe I, Telford SR, 3rd, Menon S, Brauns T, Spielman A, Gelfand JA, Wortis HH. Age-associated decline in resistance to Babesia microti is genetically determined. J Infect Dis. 2004;189:1721–1728. doi: 10.1086/382965. [DOI] [PubMed] [Google Scholar]
- Waldmann H, Munro A. The inter-relationship of antigenic structure, thymus-independence and adjuvanticity. IV. A general model for B-cell induction. Immunology. 1975;28:509–522. [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Brandes JC, Schmidt D, Fulbright JW, Goronzy JJ. Functional properties of CD4+ CD28− T cells in the aging immune system. Mech Ageing Dev. 1998;102:131–147. doi: 10.1016/s0047-6374(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- Wrann CD, Tabriz NA, Barkhausen T, Klos A, van Griensven M, Pape HC, Kendoff DO, Guo R, Ward PA, Krettek C, Riedemann NC. The phosphatidylinositol 3-kinase signaling pathway exerts protective effects during sepsis by controlling C5a-mediated activation of innate immune functions. J Immunol. 2007;178:5904–5908. doi: 10.4049/jimmunol.178.9.5940. [DOI] [PubMed] [Google Scholar]
- Yoon P, Keylock KT, Hartman ME, Freund GG, Woods JA. Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev. 2004;125:137–143. doi: 10.1016/j.mad.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Yu Y, Nagai S, Wu H, Neish AS, Koyasu S, Gewirtz AT. TLR5-mediated phosphoinositide 3-kinase activation negatively regulates flagell-ininduced proinflammatory gene expression. J Immunol. 2006;176:6194–6201. doi: 10.4049/jimmunol.176.10.6194. [DOI] [PubMed] [Google Scholar]


