Abstract
Biological membranes consist of lipid bilayers. The lipid compositions between the two leaflets of the plasma membrane differ, generating lipid asymmetry. Maintenance of proper lipid asymmetry is physiologically quite important, and its collapse induces several cellular responses including apoptosis and platelet coagulation. Thus, a change in lipid asymmetry must be restored to maintain “lipid asymmetry homeostasis.” However, to date no lipid asymmetry-sensing proteins or any related downstream signaling pathways have been identified. We recently demonstrated that expression of the putative yeast sphingoid long-chain base transporter/translocase Rsb1 is induced when glycerophospholipid asymmetry is altered. Using mutant screening, we determined that the pH-responsive Rim101 pathway, the protein kinase Mck1, and the transcription factor Mot3 all act in lipid asymmetry signaling, and that the Rim101 pathway was activated in response to a change in lipid asymmetry. The activated transcription factor Rim101 induces Rsb1 expression via repression of another transcription repressor, Nrg1. Changes in lipid asymmetry are accompanied by cell surface exposure of negatively charged phospholipids; we speculate that the Rim101 pathway recognizes the surface charges.
INTRODUCTION
Biological membranes are composed of lipid bilayers, and in the plasma membrane the lipid compositions differ between the two leaflets, resulting in asymmetry. Glycerophospholipids and sphingolipids contribute greatly to such asymmetry. Phosphatidylcholine (PC) and complex sphingolipids, including sphingomyelin (SM) and glycosphingolipids in mammals and myo-inositol–containing sphingolipids in the yeast Saccharomyces cerevisiae, are located mainly in the outer (extracytosolic) leaflet. Conversely, phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylinositol (PI) are confined to the inner (cytosolic) leaflet (Pomorski et al., 2004; Ikeda et al., 2006). The hydrophilic nature of the headgroups of these amphiphilic lipids hinders their ability to traverse the hydrophobic membrane interior, and spontaneous flip-flop of the protein-free model membrane occurs only in low frequency (Bai and Pagano, 1997). However, situated in the biological membranes are enzymes called lipid translocases or flippases, which catalyze the transbilayer movement of lipids and establish their asymmetry. Recent studies have identified three classes of translocases/transporters for glycerophospholipids: ABC transporters, P-type ATPases, and scramblases (Holthuis and Levine, 2005; Ikeda et al., 2006). ABC transporters catalyze flop, which is the movement from the cytosolic to extracytosolic leaflet, whereas P-type ATPases stimulate flip, the reverse movement. Scramblases randomize the lipid distribution between the two leaflets. Sometimes, discriminating between a translocase and transporter is difficult. For example, it is unclear whether a lipid in one leaflet is directly translocated to the other leaflet or is first transported to the other side of the hydrophilic fluid and then reincorporated into the membrane.
Maintenance of proper lipid asymmetry is important for several membrane functions. For instance, in mammals skeletal proteins like spectrin improve the mechanical stability of red blood cells by interacting with PS in the inner leaflet (Manno et al., 2002). Similarly, when certain glycerophospholipid translocase genes (DNF1, DNF2, DNF3, and DRS2) are deleted in yeast, intracellular trafficking and maintenance of organelle structure are impaired (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002; Pomorski et al., 2003; Natarajan et al., 2004; Saito et al., 2004; Furuta et al., 2007). On the other hand, local or global changes in lipid asymmetry can induce several cellular responses. PS exposed on the outer leaflet of apoptotic cells, as a result of membrane collapse, is used as a recognition signal by phagocytes (Fadok et al., 1992). PS exposure on activated platelets is also essential for blood coagulation (Zwaal et al., 1998; Lentz, 2003). In addition, transient PE exposure and loss of cell surface SM have been observed at cleavage furrows during cytokinesis, and the interaction of exposed PE with PE-binding compounds results in cell cycle arrest (Emoto et al., 1996).
Some, but not all, ABC transporters catalyze the flop of glycerophospholipids (Ikeda et al., 2006). In mammals, translocation/transport of glycerophospholipids (with little selectivity) by ABCB1 (MDR1) has been reported (van Helvoort et al., 1996), as has that of PC by ABCB4 (human MDR3, mouse mdr2; Smit et al., 1993), PS by ABCA1 (Abramova et al., 2001), and N-retinylidene-PE by ABCA4 (Weng et al., 1999). In yeast, Pdr5 and Yor1 are thought to be involved in the flop of glycerophospholipids (Decottignies et al., 1998; Pomorski et al., 2003). PDR5 and YOR1 are regulated by the transcription factor Pdr1, and their up-regulation in a gain-of-function PDR1-3 mutant causes a reduction in the accumulation of labeled PE, probably due to increased efflux (Decottignies et al., 1998). A Pdr5/Yor1-dependent increase in endogenous PE on the cell surface was later confirmed (Pomorski et al., 2003).
A subfamily of P-type ATPases (type 4 subfamily or amino-phospholipid translocases) mediates the flip of glycerophospholipids. In mammals, ATP8A1, ATP8B1, and ATP8B3 are reportedly involved in PS translocation (Ujhazy et al., 2001; Wang et al., 2004; Paterson et al., 2006). In yeast, five proteins belong to this family, Dnf1 and Dnf2 in the plasma membrane and Neo1, Drs2, and Dnf3, which reside in the internal membranes (Pomorski et al., 2003). Disruption of the DNF1 and DNF2 genes abolishes the ATP-dependent flip of fluorescent-labeled PC, PE, and PS as well as any reduction in cell surface PE, indicating that Dnf1 and Dnf2 have important roles in maintaining the glycerophospholipid asymmetry of the plasma membrane (Pomorski et al., 2003). To properly target the plasma membrane, Dnf1 and Dnf2 require association with a common subunit, Lem3 (also known as Ros3), a member of the Cdc50 family (Saito et al., 2004; Furuta et al., 2007). Therefore, a lem3Δ mutant exhibits effects similar to those of a dnf1Δ dnf2Δ double mutant, i.e., defective accumulation of exogenously added fluorescent-labeled PC or PE (Kato et al., 2002; Hanson et al., 2003).
Although sphingolipids also contribute to lipid asymmetry formation, knowledge of sphingolipid translocases is limited. In yeast, we identified Rsb1 (Yor049c) as a putative sphingoid long-chain base-specific translocase/transporter (Kihara and Igarashi, 2002). Rsb1 apparently mediates the flop or efflux of long-chain base in an ATP-dependent manner (Kihara and Igarashi, 2002, 2006). Rsb1 expression is low in wild-type cells under normal growth conditions. However, its expression is significantly induced when glycerophospholipid asymmetry is altered, such as by mutations in genes involved in either the flip (P-type ATPases DNF1 and DNF2 or the regulatory subunit LEM3) or the flop (ABC transporters PDR5 and YOR1) of glycerophospholipids (Kihara and Igarashi, 2004). Conversely, Rsb1 overproduction promotes the flip and represses the flop of fluorescent-labeled PC and PE (Kihara and Igarashi, 2004). This suggests the existence of cross-talk between glycerophospholipids and sphingolipids in lipid asymmetry formation and of sensor molecules that detect changes in glycerophospholipid asymmetry or in downstream signal transduction pathways leading to Rsb1 expression. However, information regarding such factors and pathways is completely lacking.
To identify factors required for Rsb1 induction, we screened for mutants having defects in the Rsb1 expression normally induced by alternations in lipid asymmetry. This screening identified five genes (MCK1, MOT3, RIM13, RIM20, and RIM21) in addition to the previously characterized gene PDR1. Of these genes, three (RIM13, RIM20, and RIM21) are known to be involved in the alkaline pH-responsive Rim101 pathway. Further analyses revealed that the Rim101 pathway is indispensable for the induction of Rsb1. Thus, our findings provide new insight into signaling associated with lipid asymmetry, which is the convergence of lipid asymmetry and alkaline pH adaptation through the Rim101 pathway.
MATERIALS AND METHODS
Yeast Strains and Media
S. cerevisiae strains used are listed in Table 1. Cells were grown in either YPD medium (1% yeast extract, 2% bactopeptone, and 2% d-glucose) or synthetic complete (SC) medium (0.67% yeast nitrogen base and 2% d-glucose) containing nutritional supplements. Buffered SC was prepared using 0.1 M sodium citrate buffers (pH 4.4 and 5.4) or 0.1 M sodium phosphate buffers (pH 6.4 and 7.4). The tetracyclic peptide antibiotic Ro 09-0198 (cinnamycin), kindly provided by Dr. Masato Umeda (Kyoto University, Japan), was dissolved in dimethyl sulfoxide/water, 1:1 (vol/vol). Ro 09-0198 and the peptide-derived drug proteasome inhibitor MG132 (carbobenzoxyl-leucinyl-leucinyl-leucinal; Sigma, St. Louis, MO) were diluted in YPD medium for use in drug sensitivity assays.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| SEY6210 | MATα leu2-3, 112 ura3-52 his3Δ200 trp1Δ901 lys2-801 suc2Δ9 | Robinson et al. (1988) |
| KCY72 | SEY6210, rsb1Δ::URA3 ade2Δ::TRP1 | This study |
| KCY86 | SEY6210, rsb1Δ::PRSB1-ADE2 ade2Δ::TRP1 | This study |
| KCY113 | SEY6210, rsb1Δ::PRSB1-ADE2 ade2Δ::TRP1 pdr5Δ::URA3 | This study |
| KCY594 | SEY6210, nrg1Δ::KanMX4 | This study |
| KCY595 | SEY6210, rim101Δ::KanMX4 | This study |
| KCY662 | SEY6210, rsb1::RSB1-HA TRP1 | This study |
| KCY689 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 | This study |
| KCY692 | SEY6210, rsb1::RSB1-HA TRP1 lem3Δ::HIS3 | This study |
| KCY694 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 pdr1Δ::LEU2 | This study |
| KCY696 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 lem3Δ::HIS3 | This study |
| KCY697 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim20Δ::KanMX4 | This study |
| KCY1011 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 yor1Δ::KanMX4 | This study |
| KCY1012 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 mck1Δ::KanMX4 | This study |
| KCY1013 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 mot3Δ::KanMX4 | This study |
| KCY1014 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim13Δ::KanMX4 | This study |
| KCY1015 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim21Δ::KanMX4 | This study |
| KCY1016 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim101Δ::KanMX4 | This study |
| KCY1029 | SEY6210, rsb1::RSB1-HA TRP1 dnf1Δ::KanMX4 dnf2Δ::URA3 | This study |
| KCY1046 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 dfg16Δ::KanMX4 | This study |
| KCY1047 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim9Δ::KanMX4 | This study |
| KCY1048 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim8Δ::KanMX4 | This study |
| KCY1049 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 ygr122wΔ::KanMX4 | This study |
| KCY1051 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 vps28Δ::KanMX4 | This study |
| KCY1052 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 vps25Δ::KanMX4 | This study |
| KCY1053 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 snf7Δ::KanMX4 | This study |
| KCY1054 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 vps20Δ::KanMX4 | This study |
| KCY1055 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 did4Δ::KanMX4 | This study |
| KCY1056 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 vps24Δ::KanMX4 | This study |
| KCY1060 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 ime1Δ::KanMX4 | This study |
| KCY1061 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 nrg1Δ::KanMX4 | This study |
| KCY1062 | SEY6210, rsb1::RSB1-HA TRP1 lem3Δ::HIS3 mck1Δ::KanMX4 | This study |
| KCY1063 | SEY6210, rsb1::RSB1-HA TRP1 lem3Δ::HIS3 mot3Δ::KanMX4 | This study |
| KCY1064 | SEY6210, rsb1::RSB1-HA TRP1 lem3Δ::HIS3 rim101Δ::KanMX4 | This study |
| KCY1065 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 mot3Δ::KanMX4 mck1Δ::LEU2 | This study |
| KCY1066 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 mot3Δ::KanMX4 rim101Δ::LEU2 | This study |
| KCY1067 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 rim101Δ::KanMX mck1Δ::LEU2 | This study |
| KCY1070 | SEY6210, rsb1::RSB1-HA TRP1 pdr5Δ::URA3 nrg1Δ::KanMX4 rim101Δ::LEU2 | This study |
| KCY1102 | SEY6210, rsb1::RSB1-HA TRP1 mck1Δ::KanMX4 | This study |
| KCY1103 | SEY6210, rsb1::RSB1-HA TRP1 mot3Δ::KanMX4 | This study |
| KCY1104 | SEY6210, rsb1::RSB1-HA TRP1 rim101Δ::KanMX4 | This study |
| KCY1112 | SEY6210, rsb1::RSB1-HA TRP1 URA3 | This study |
| KCY1141 | SEY6210, rsb1::RSB1-HA TRP1 lem3Δ::KanMX4 URA3 | This study |
The chromosomal RSB1 gene was tagged at its 3′-terminus with three copies of a hemagglutinin (HA) epitope. This was achieved by replacing the RSB1 gene with a fragment containing both the RSB1-HA and a TRP1 marker by essentially the same method described previously (Uemura et al., 2007).
Plasmids
Primers and templates used are listed in Tables 2 and 3. The pFI1 plasmid (Hayashi et al., 2005), which encodes N-terminally triple HA-tagged Rim101 (HA-Rim101), was a kind gift from Dr. Tatsuya Maeda (Tokyo University, Japan). The pIKD412 plasmid, which is derived from the pRS423 vector (2 μ, HIS3 marker) (Christianson et al., 1992), encodes RSB1-HA under the control of the RSB1 promoter (PRSB1). Three putative Nrg1-binding sites in pIKD412 were mutated by site-direct mutagenesis using a QuikChange kit (Stratagene, La Jolla, CA).
Table 2.
Primers used in this study
| Primer | Nucleotide sequence |
|---|---|
| ADE2-3 | 5′-CAGCTGATGGATTCTAGAACAGTTGGTATATTAG-3′ (PvuII) |
| ADE2-4 | 5′-GGATCCTTACTTGTTTTCTAGATAAGCTTCGTAAC-3′ (BamHI) |
| RIM101-3 | 5′-GGATCCGTGCCATTGGAAGATCTGCTTAAT-3′ (BamHI) |
| RIM101-4 | 5′-TCATACCAAAATTTTGGGATACTTGG-3′ |
| RIM101-5 | 5′-CTGGGAATTTAGCCTGAACTAGTCAAAAAAGCTGTACTAATG-3′ |
| RIM101-6 | 5′-CATTAGTACAGCTTTTTTGACTAGTTCAGGCTAAATTCCCAG-3′ |
| RSB1-15 | 5′-AACAAAACTCTCCGAGATTTCAATCCAGAGCATAG-3′ (mt1) |
| RSB1-16 | 5′-CTATGCTCTGGATTGAAATCTCGGAGAGTTTTGTT-3′ (mt1) |
| RSB1-17 | 5′-CACGGCCATGCCTTGATTTTTCTGTCGCCCCCTAG-3′ (mt2) |
| RSB1-18 | 5′-CTAGGGGGCGACAGAAAAATCAAGGCATGGCCGTG-3′ (mt2) |
| RSB1-19 | 5′-TGAGGGTTCTGTCGCAAACTAGCTGTACGTAAGC-3′ (mt3) |
| RSB1-20 | 5′-GCTTACGTACAGCTAGTTTGCGACAGAACCCTCA-3′ (mt3) |
| RSB1-21 | 5′-TGATTTTTCTGTCGCAAACTAGCTGTACGTAAGC-3′ (mt3) |
| RSB1-22 | 5′-GCTTACGTACAGCTAGTTTGCGACAGAAAAATCA-3′ (mt3) |
| RSB1-33 | 5′-ATACGACTCACTATAGGGCGAATTGG-3′ |
| RSB1-34 | 5′-TGCGTTCTCGAGTTTGAATTTCTCAACGTCTATAA-3′ (XhoI) |
Underlined letters are the restriction sites created or the mutated nucleotides.
Table 3.
Primers and templates used for construction of NRE-mutated plasmids
| Plasmid | Primers | Template |
|---|---|---|
| pIKD414 (PRSB1(mt1)-RSB1-HA) | RSB1-15 and RSB1-16 | pIKD412 |
| pIKD416 (PRSB1(mt2)-RSB1-HA) | RSB1-17 and RSB1-18 | pIKD412 |
| pIKD418 (PRSB1(mt3)-RSB1-HA) | RSB1-19 and RSB1-20 | pIKD412 |
| pIKD420 (PRSB1(mt2/3)-RSB1-HA) | RSB1-21 and RSB1-22 | pIKD416 |
The pIKD509 (PTDH3-Myc-RIM101-531) plasmid, which encodes a constitutively active form of Rim101, was constructed as follows. The RIM101 gene was amplified using genomic DNA prepared from SEY6210 cells and the primers RIM101-3 and RIM101-4 (Table 2). The resulting fragment was cloned into a pGEM-T Easy (Promega, Madison, WI) vector to generate the pAD21 plasmid. The 1.9-kb BamHI-NotI region of pAD21 was then cloned into the BamHI-NotI site of pAK303, which is a derivative of the pRS315 vector (CEN, LEU2 marker) (Sikorski and Hieter, 1989) designed to produce an N-terminal Myc-tagged protein under the control of the TDH3 promoter (PTDH3), generating the pIKD432 plasmid. A stop codon was then inserted between codons 531 and 532 by site-direct mutagenesis using a QuikChange kit (Stratagene) and primers RIM101-5 and RIM101-6, creating pIKD509.
For use in the β-galactosidase (LacZ) assay, the pIKD493 (PRSB1-lacZ) plasmid was constructed as follows. The pBgal-Basic plasmid (Clontech, Mountain View, CA), which encodes the lacZ gene, was digested with SgrAI, blunted using KOD polymerase (Toyobo, Osaka, Japan), and digested further with XhoI. The resulting fragment was cloned into the XhoI-SmaI site of the pRS423 vector, producing the pIKD491 plasmid. The RSB1 promoter from the pIKD412 plasmid was then amplified using primers RSB1-33 and RSB1-34 (Table 2), and its XhoI-XhoI fragment was inserted into the XhoI site of the pIKD491 plasmid, creating the pIKD493 plasmid.
Screening for Mutants Defective in Lipid Asymmetry Signaling
To identify genes coding for the presumed lipid asymmetry-sensing factor and/or factors involved in its downstream signaling pathways, we screened for mutants exhibiting defects in change-induced Rsb1 expression. For this purpose, the RSB1 gene was replaced with ADE2, which codes for a protein involved in the biosynthesis of purine nucleotides, although the RSB1 promoter was left intact. Reduced Ade2 levels cause an accumulation of a red purine precursor, making the colony appear red, and, in this case, reflecting the RSB1 promoter activity. KCY113 (pdr5Δ PRSB1-ADE2) cells, used in the mutant screening, were constructed as follows.
The ADE2 gene was amplified by PCR using genomic DNA prepared from SEY6210 cells as a template and the primers ADE2-3 and ADE2-4 (Table 2). The amplified fragment was cloned into pGEM-T Easy, creating the pIKD248 plasmid. The 0.9-kb HpaI-BclI HA-RSB1 region of the pAK464 plasmid (Kihara and Igarashi, 2004), which contains PRSB1-HA-RSB1–3′-UTRRSB1, was then replaced by the 1.7-kb PvuII-BamHI ADE2 fragment of pIKD248, creating pIKD249. The PRSB1-ADE2-3′-UTRRSB1 fragment of pIKD249 was then introduced into KCY72 cells. Cells undergoing homologous recombination between PRSB1-ADE2-3′-UTRRSB1 and rsb1Δ::URA3 were expected to lose the URA3 marker, so we selected such cells with 50 μg/ml 5-fluoro-orotic acid. One of the selected clones, KCY86 exhibited the proper genotype and were used in this study. KCY113 (pdr5Δ PRSB1-ADE2) cells were then constructed by introducing the pdr5Δ::URA3 mutation into the KCY86 cells.
Screening of mutants defective in lipid asymmetry signaling was performed using a genomic library (kindly provided by Dr. Michael Snyder, Yale University, New Haven, CT) that had been mutagenized by random insertion of the transposon mTn-lacZ/LEU2 (Burns et al., 1994). The genomic library was digested with NotI, and the resulting DNA fragments were transformed into KCY113 cells. Pooled transposon-carrying mutants were plated at ∼2.0 × 103 cells per plate on YPD plates. The plates were then incubated at 30°C for 2 d. Mutants exhibiting a darker red than that of KCY113 cells were obtained at a frequency of ∼1/1400. Of these, we chose 15 mutants for further analysis. The sites of transposon insertion in the isolated mutants were determined according to the manuals of the Yale Genome Analysis Center (http://ygac.med.yale.edu/).
Immunoblotting
Yeast cells were precultured overnight in YPD medium at 30°C and then diluted into YPD medium to 0.3 OD600 unit/ml. Cells bearing plasmids were precultured in SC medium instead of YPD medium. After being grown at 30°C to ∼1 OD600 unit/ml, the cells were collected. Preparation of total cell lysate and immunoblotting were performed as described previously (Kihara and Igarashi, 2002). Protein concentrations were measured using a BCA protein assay reagent (Pierce, Rockford, IL). Immunoblotting was performed using as primary antibodies anti-HA (Y-11; 0.16 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Myc (PL-14; 1 μg/ml; Medical and Biological Laboratories, Nagoya, Japan), and anti-phosphoglycerokinase 1 (Pgk1; 22C5; 0.0625 μg/ml; Molecular Probes, Eugene, OR) antibodies. HRP-conjugated anti-rabbit or anti-mouse IgG F(ab′)2 (each from GE Healthcare Bio-Sciences, Piscataway, NJ; diluted 1:10,000) was used as the secondary antibody. Labeling was detected using an enhanced chemiluminescence (ECL) or ECL plus kit (GE Healthcare Bio-Sciences). For detecting HA-Rim101, Myc-Rim101-531, and genomic-encoded Rsb1-HA proteins, we enhanced the signal of the anti-HA antibody Y-11 and the anti-Myc antibody PL-14 using Can Get Signal Immunoreaction Enhancer Solution (Toyobo), according to the manufacturer's manual.
Deglycosylation
Proteins (5 μl) in 1× SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and a trace amount of bromophenol blue containing 5% 2-mercaptoethanol) were diluted with 40 μl 0.05 M sodium citrate (pH 5.5) containing 1 mM phenylmethylsulfonyl fluoride, and treated at 37°C for 1 h with 1000 U of recombinant endoglycosidase H (Endo H; New England Biolabs, Beverly, MA). The samples were then mixed with 12 μl 4× SDS sample buffer and 3 μl 2-mercaptoethanol and incubated at 37°C for 5 min. A portion of each sample was separated by SDS-PAGE and subjected to immunoblotting.
β-Galactosidase Assay
β-Galactosidase activities were determined as described elsewhere (Simon and Lis, 1987), with minor modifications. Cells bearing the pIKD493 plasmid (PRSB1-lacZ) were precultured in SC medium lacking histidine then diluted into 5 ml YPD medium. After a 4-h incubation at 30°C, the cells were washed with 1 ml buffer I (10 mM Tris-HCl, pH 7.5, and 5 mM dithiothreitol) and suspended in 50 μl buffer I. Acid-washed glass beads (Sigma) were added to the cells, and the cells were broken by mixing vigorously for 15 min at 4°C. Another 50 μl of buffer I was then added to the cells, and the samples were mixed vigorously for 5 min. Cell debris was removed by centrifugation, and the supernatants were subjected to a β-galactosidase assay. Before initiating the reaction, 144 μl assay buffer (50 mM potassium phosphate, pH 7.5, and 1 mM MgCl2) and 3 μl of 50 mM chlorophenol red-β-d-galactopyranoside (the substrate) were mixed and incubated at 37°C. The reaction was started by adding 3 μl cell lysate to the above substrate solution. After a 6-min incubation at 37°C, the reaction was terminated by adding 250 μl 1 M Na2CO3, and the β-galactosidase activity was examined by measuring the OD574. The protein concentrations of the cell lysates were measured using a Coomassie (Bradford) protein assay reagent (Pierce). β-Galactosidase activity was normalized to the protein concentration, and the activity of KCY662 (wild-type) cells harboring the pRS423 vector was subtracted as a background. One unit of β-galactosidase was defined as the amount needed per minute to degrade 1 mmol of the substrate chlorophenol red-β-d-galactopyranoside to chlorophenol red and d-galactose.
RESULTS
Accumulation of PE in the Outer Leaflet of pdr5Δ and pdr5Δ yor1Δ Mutants
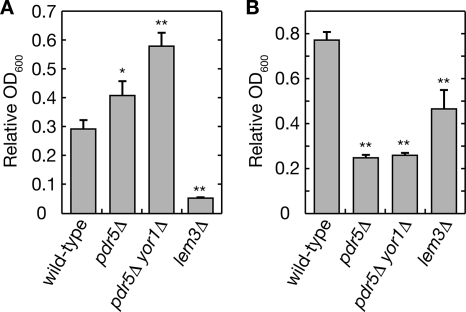
We previously demonstrated that Rsb1 expression is induced in yeast cells carrying the floppase mutation pdr5Δ and is further enhanced with the pdr5Δ yor1Δ double mutation (Kihara and Igarashi, 2004). Because up-regulation of Pdr5 and Yor1 in a gain-of-function PDR1-3 mutant results in a reduction in the amount of PE exposed on the cell surface (Decottignies et al., 1998), we considered that altered lipid asymmetry resulting from the pdr5Δ and pdr5Δ yor1Δ mutations induced the Rsb1 expression. However, to date the effects of the pdr5Δ and yor1Δ mutations have not been examined in a wild-type background (PDR1+) or in our strain background. Therefore, we investigated the amount of surface-exposed PE in our pdr5Δ and pdr5Δ yor1Δ cells using the tetracyclic peptide antibiotic Ro 09-0198 (Ro), which specifically binds to PE (Wakamatsu et al., 1990; Umeda and Emoto, 1999). Ro binding to PE results in cytolysis (Aoki et al., 1994; Kato et al., 2002), so the amount of surface-exposed PE can easily be estimated by measuring the overall sensitivity of the cell to Ro. As shown in Figure 1A, treatment of wild-type cells with 50 μM Ro caused a reduction in their growth rate (to ∼30%). As expected, pdr5Δ cells were more resistant to Ro than were wild-type cells, exhibiting a reduced rate of only 40%. Moreover, introduction of the yor1Δ mutation into the pdr5Δ cells further enhanced the Ro tolerance. This tolerance may be underestimated, though, because the pdr5Δ and pdr5Δ yor1Δ mutants tend to accumulate several drugs intracellularly unlike wild-type cells, probably because of diminished ability to pump them out (Leonard et al., 1994; Máhe et al., 1996; Decottignies et al., 1998). Indeed, the pdr5Δ and pdr5Δ yor1Δ mutants were more sensitive to another peptide-derived drug, the proteasome inhibitor MG132, than were wild-type cells (Figure 1B), consistent with results reported by others (Fleming et al., 2002).
Figure 1.
Cell surface PE is reduced in cells carrying the pdr5Δ mutation. KCY1112 (wild-type), KCY689 (pdr5Δ), KCY1011 (yor1Δ pdr5Δ), and KCY1141 (lem3Δ) cells were diluted to 0.05 OD600 unit/ml in YPD medium containing 50 μM Ro (A) or 200 μM MG132 (B). After a 9-h incubation, the OD600 of each culture was measured. Values indicate the OD600 for each cell line in the presence of the drugs, relative to that in the absence of the drugs. Values represent the means ± SD from three independent experiments. The statistical significance of each difference as compared with results from wild-type cells was determined using a two-tailed Student's t test. *p < 0.05; **p < 0.01.
In contrast to these floppase mutants, cells carrying the flippase mutant lem3Δ were highly sensitive to Ro (Figure 1A), agreeing with a previous report (Kato et al., 2002). The lem3Δ mutant was also more sensitive to MG132 than were wild-type cells, suggesting that increased permeability of the cell membrane may contribute in part to the sensitivity, in addition to increased PE exposure. These results confirm that the amount of surface-exposed PE is lower in pdr5Δ yor1Δ, and pdr5Δ cells, respectively, than in wild-type cells.
Isolation of Mutants Defective in Rsb1 Expression Induced by Changes in Lipid Asymmetry
To identify genes coding for the presumed lipid asymmetry-sensing factor and/or factors involved in its downstream signaling pathways, we screened for mTn-lacZ/LEU2 transposon-inserted mutants unable to activate RSB1 promoter-dependent expression in response to a mutation in the PDR5 gene. This screening identified five genes (MCK1, MOT3, RIM13, RIM20, and RIM21) in addition to the previously characterized gene PDR1 (Kihara and Igarashi, 2004). Of these genes, three (RIM13, RIM20, and RIM21) are known to be involved in the pH-responsive Rim101 pathway (Peñalva and Arst, 2004). MOT3 is a transcription factor gene involved in the repression of anaerobic condition–induced genes (Abramova et al., 2001; Hongay et al., 2002) and MCK1 encodes a serine/threonine/tyrosine kinase, which shares similarity with kinases of the mammalian glycogen synthase kinase 3 subfamily (Lim et al., 1993).
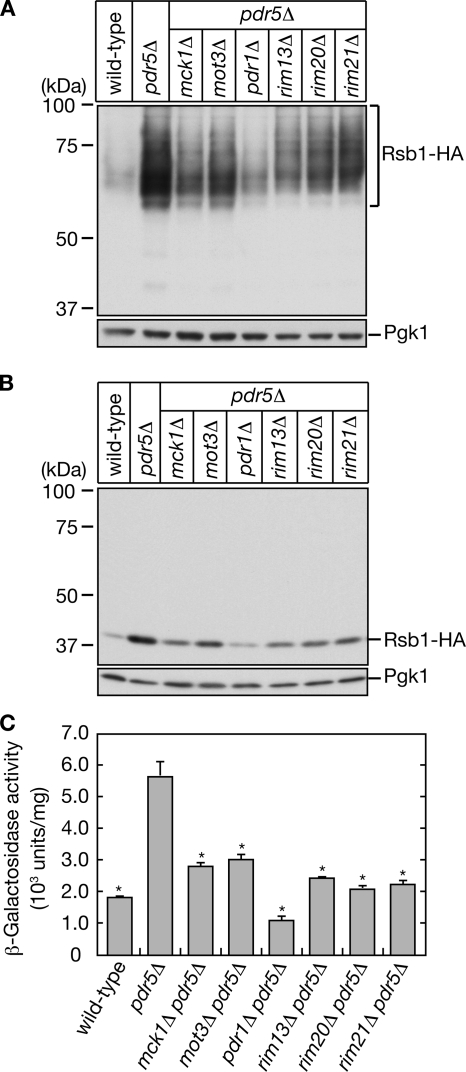
To confirm that the isolated transposon mutations were indeed responsible for the Rsb1 induction, we introduced a deletion mutation of each of the isolated genes into KCY689 (pdr5Δ RSB1-HA) cells, in which the chromosomal RSB1 gene has been C-terminally triple HA-tagged (RSB1-HA). Rsb1 is an N-glycosylated protein (Panwar and Moye-Rowley, 2006) and as such was detected in immunoblots as a broad band of 57-90 kDa (Figure 2A), which was shifted to a single 41-kDa band upon treatment with Endo H (Figure 2B). Regardless of the level of glycosylation, pdr5Δ-induced Rsb1-HA protein expression was indeed reduced by all the mutations tested (Figure 2, A and B). The pdr1Δ mutation had the most prominent effect. The mck1Δ, rim13Δ, rim20Δ, and rim21Δ mutations caused reduced expression to a similar extent (Figure 2, A and B).
Figure 2.
MCK1, MOT3, RIM13, RIM20, and RIM21 are involved in Rsb1 expression induced by changes in lipid asymmetry. KCY662 (wild-type), KCY689 (pdr5Δ), KCY1012 (mck1Δ pdr5Δ), KCY1013 (mot3Δ pdr5Δ), KCY694 (pdr1Δ pdr5Δ), KCY1014 (rim13Δ pdr5Δ), KCY697 (rim20Δ pdr5Δ), and KCY1015 (rim21Δ pdr5Δ) cells were grown in YPD medium. (A and B) Total proteins were prepared from each culture. (A) Proteins (13 μg) were separated by SDS-PAGE and then subjected to immunoblotting with an anti-HA antibody or, to demonstrate uniform protein loading, an anti-Pgk1 antibody. (B) Proteins (1.7 μg) were treated with Endo H, separated by SDS-PAGE, and subjected to immunoblotting as in A. (C) Total cell lysates were prepared from each culture and subjected to β-galactosidase reporter assays. Values indicate the means ±SD from three independent experiments. The statistical significance of each difference as compared with results from pdr5Δ cells was determined using a two-tailed Student's t test. *p < 0.01.
Because our screening method was designed to identify genes involved in the regulation of transcription from the RSB1 promoter, the isolated mutations must affect Rsb1 expression at the transcriptional level rather than at the posttranslational level, such as by enhanced protein degradation. To confirm this, we performed a β-galactosidase (LacZ) assay using cells in which the expression of LacZ was under the control of the RSB1 promoter. Consistent with the above result, all the mutations tested repressed pdr5Δ-induced β-galactosidase activity. Again the pdr1Δ mutation exhibited the most pronounced effect (Figure 2C).
The Rim101 Pathway Is Involved in the Induction of Rsb1
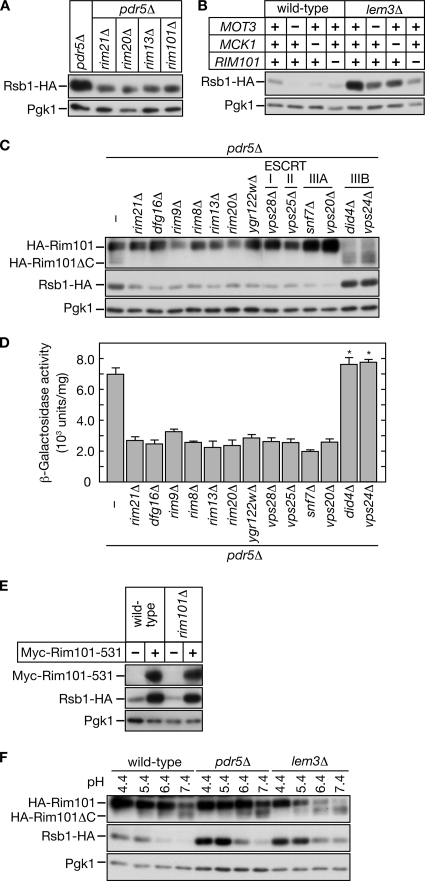
In the pH-responsive pathway, Rim13, Rim20, and Rim21 are all located upstream of the transcription factor Rim101 (Hayashi et al., 2005; Boysen and Mitchell, 2006). Rim21 and Dfg16, both multimembrane-spanning proteins, and their homologues are thought to serve as pH sensors (Peñalva and Arst, 2004; Barwell et al., 2005; Herranz et al., 2005; Boysen and Mitchell, 2006). An alkaline signal activates Rim101 via proteolytic processing of its C-terminus by the calpain-like cysteine protease Rim13, with assistance from Rim20 (Li and Mitchell, 1997; Futai et al., 1999; Xu and Mitchell, 2001). Truncated Rim101 then enters the nucleus and modulates the expressions of pH-responsive genes. To investigate whether Rim101 is involved in the induction of Rsb1, a rim101Δ mutation was introduced into KCY689 (pdr5Δ PRSB1-RSB1-HA) cells. The rim101Δ mutation reduced the Rsb1-HA expression to a similar extent as the rim21Δ, rim20Δ, and rim13Δ mutations (Figure 3A). This suggests that Rim21, Rim20, and Rim13 regulate the Rsb1 expression via Rim101.
Figure 3.
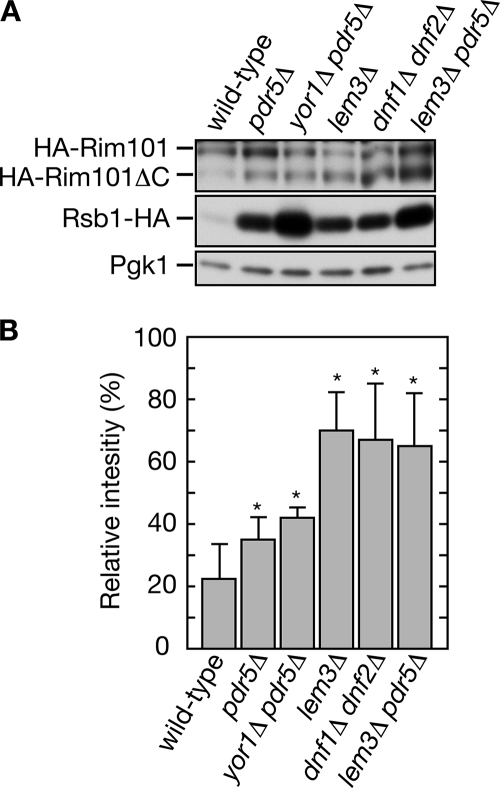
The pH-responsive Rim101 pathway is involved in lipid asymmetry signaling. (A and B) Cells carrying single or double mutations were grown in YPD medium. Total proteins (1.7 μg) prepared from each culture were incubated with Endo H, separated by SDS-PAGE, and subjected to immunoblotting with an anti-HA or anti-Pgk1 antibody. (A) KCY689 (pdr5Δ), KCY1014 (rim13Δ pdr5Δ), KCY697 (rim20Δ pdr5Δ), KCY1015 (rim21Δ pdr5Δ), and KCY1016 (rim101Δ pdr5Δ) cells were used. (B) KCY662 (wild-type), KCY1103 (mot3Δ), KCY1102 (mck1Δ), KCY1104 (rim101Δ), KCY692 (lem3Δ), KCY1063 (mot3Δ lem3Δ), KCY1062 (mck1Δ lem3Δ), and KCY1064 (rim101Δ lem3Δ) cells were used. (C and D) KCY689 (pdr5Δ), KCY1015 (rim21Δ pdr5Δ), KCY1046 (dfg16Δ pdr5Δ), KCY1047 (rim9Δ pdr5Δ), KCY1048 (rim8Δ pdr5Δ), KCY1014 (rim13Δ pdr5Δ), KCY697 (rim20Δ pdr5Δ), KCY1049 (ygr122wΔ pdr5Δ), KCY1051 (vps28Δ pdr5Δ), KCY1052 (vps25Δ pdr5Δ), KCY1053 (snf7Δ pdr5Δ), KCY1054 (vps20Δ pdr5Δ), KCY1055 (did4Δ pdr5Δ), and KCY1056 (vps24Δ pdr5Δ) cells bearing the pFI1 (HA-RIM101) or pIKD493 (PRSB1-lacZ) plasmid were used. (C) Cells harboring the plasmid pFI1 were precultured in SC medium lacking leucine, transferred to YPD medium, and grown to logarithmic phase. Total proteins were prepared from each culture and incubated with Endo H. Proteins (1.25 μg for Rsb1-HA and Pgk1 blots, and 1.7 μg for HA-Rim101 blots) were separated by SDS-PAGE and subject to immunoblotting with an anti-HA or anti-Pgk1 antibody. (D) Cells harboring the plasmid pIKD493 were precultured in SC medium lacking histidine, transferred to YPD medium, and grown to logarithmic phase. Total cell lysates were prepared from each culture and subjected to β-galactosidase reporter assays. Values represent the means ± SD from three independent experiments. The statistical significance of each difference as compared with results from the pdr5Δ cells bearing pIKD493 was determined using a two-tailed Student's t test. (* p < 0.05) (E) KCY662 (wild-type) and KCY1104 (rim101Δ) cells harboring the pRS315 or pIKD509 (Myc-RIM101-531) plasmid were cultured in SC medium lacking leucine and transferred to YPD medium. Total proteins prepared from each culture were incubated with Endo H. Proteins (2.5 μg for Myc-Rim101-531 blots and 1.25 μg for Rsb1-HA and Pgk1 blots) were separated by SDS-PAGE and subjected to immunoblotting with an anti-Myc, anti-HA, or anti-Pgk1 antibody. (F) KCY662 (wild-type), KCY689 (pdr5Δ), and KCY692 (lem3Δ) cells, each bearing the pFI1 plasmid, were cultured in SC medium lacking leucine for 3 h. An equal volume of buffered SC medium lacking leucine was added to the culture medium, and the cells were incubated for another 2 h. Total proteins were prepared from each culture and incubated with Endo H. Proteins (1.7 μg for HA-Rim101 blots, and 1.25 μg for Rsb1-HA and Pgk1 blots) were separated by SDS-PAGE and subject to immunoblotting with an anti-HA or anti-Pgk1 antibody.
Wild-type cells express weak but detectable amounts of Rsb1, suggesting that local or transient changes in asymmetry constantly occur. We investigated the effects of mot3Δ, mck1Δ, and rim101Δ mutations on Rsb1 expression in the wild-type background. All these mutations caused decreased expression of Rsb1-HA in the wild-type background (Figure 3B) just as they had in the pdr5Δ background (Figures 2 and 3A).
Lipid asymmetry is maintained both by ABC transporter–mediated flop and by P-type ATPase-mediated flip, and a mutation in either the transporter (flop mutation; pdr5Δ or pdr5Δ yor1Δ) or the ATPase (flip mutation; lem3Δ or dnf1Δ dnf2Δ) leads to altered lipid asymmetry and induction of Rsb1 (Kihara and Igarashi, 2004). To investigate whether Rsb1 induction caused by a flip mutation would be affected by a mot3Δ, mck1Δ, or rim101Δ mutation, we introduced these mutations into KCY692 (lem3Δ PRSB1-RSB1-HA) cells. We found that all these mutations also caused reduced Rsb1-HA expression (Figure 3B). Thus, Mot3, Mck1, and the Rim101 pathway are required for the lipid asymmetry signal caused by either flip (Figure 3B) or flop mutations (Figures 2 and 3A).
Several other factors are also known to be involved in the Rim101 pathway. These include Rim8, Rim9, Dfg16, and Ygr122w, as well as certain ESCRT (endosomal sorting complex required for transport) proteins (Li and Mitchell, 1997; Xu et al., 2004; Barwell et al., 2005; Rothfels et al., 2005), which function in sorting membrane proteins to the vacuolar degradation pathway and in multivesicular body formation (Katzmann et al., 2002). To investigate which of these factors might be involved in the pdr5Δ-related Rsb1 induction, we introduced mutated versions of each gene into KCY689 (pdr5Δ PRSB1-RSB1-HA) cells and measured Rsb1-HA expression and β-galactosidase reporter activities. To monitor the activation of Rim101, triple HA-tagged Rim101 (HA-Rim101) was also expressed, and its processing was examined. As shown in Figure 3, C and D, all mutations known to impair Rim101 activation (rim21Δ, dfg16Δ, rim9Δ, rim8Δ, rim13Δ, rim20Δ, ygr122wΔ, vps28Δ, vps25Δ, snf7Δ, and vps20Δ) caused reduced Rsb1 expression. Mutations in ESCRT factors such as DID4 and VPS24 are known to cause constitutive partial activation of Rim101 (Hayashi et al., 2005). We found that these mutations caused a slight induction in Rsb1 expression (Figure 3, C and D). When a constitutive active form of Rim101 (Rim101-531), which contains a deletion at the C-terminous, was overproduced, a marked increase in Rsb1-HA amount was observed both in wild-type and rim101Δ cells (Figure 3E).
To further investigate the relationship between the Rim101 pathway and Rsb1 expression induced by changes in lipid asymmetry, we measured the Rsb1-HA in cells exposed to pH levels ranging from 4.4 to 7.4 (Figure 3F). Consistent with previous studies (Hayashi et al., 2005), Rim101 processing was enhanced at higher pH (Figure 3F). In contrast, Rsb1-HA levels were high at low pH (pH 4.4 and 5.4) but low at high pH (pH 6.4 and 7.4; Figure 3F). A similar pH-dependent decrease in Rsb1-HA expression was observed for pdr5Δ and lem3Δ cells. However, at any pH, the expression of Rsb1-HA was higher in pdr5Δ and lem3Δ cells than in wild-type cells. Exposure of cells to high pH may induce several cellular responses, any of which might function negatively in the induction of Rsb1 and surpass the effect of the activated Rim101 pathway. For example, as determined by a comprehensive microarray analysis, PDR1 mRNA is down-regulated at high pH (Causton et al., 2001).
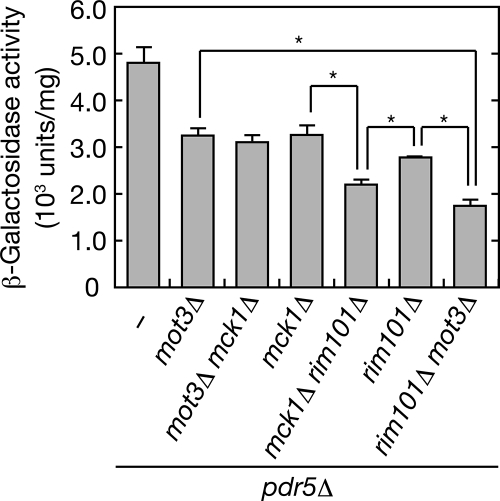
To investigate whether Mot3, Mck1, and the Rim101 pathway regulate Rsb1 expression via the same or different pathways, we generated double mutants for the MOT3, MCK1, and RIM101 genes. β-Galactosidase activitiy was additively lower in each double deletion mutant than in the corresponding single deletion mutant, except the activity in the mot3Δ mck1Δ double mutant, which was similar to that of the mot3Δ or mck1Δ single mutant (Figure 4). These results suggest that signaling through the Rim101 pathway induces Rsb1 expression independently from the Mot3/Mck1 pathway.
Figure 4.
The rim101Δ mutation and the mot3Δ or mck1Δ mutation exhibit additive effects in Rsb1 induction. KCY689 (pdr5Δ), KCY1013 (mot3Δ pdr5Δ), KCY1065 (mot3Δ mck1Δ pdr5Δ), KCY1012 (mck1Δ pdr5Δ), KCY1067 (mck1Δ rim101Δ pdr5Δ), KCY1016 (rim101Δ pdr5Δ), and KCY1066 (rim101Δ mot3Δ pdr5Δ) cells harboring the pIKD493 (PRSB1-lacZ) plasmid were grown in SC medium lacking histidine. Total cell lysates were prepared from each culture and subjected to β-galactosidase reporter assays. Values represent the means ± SD from three independent experiments. The statistical significance of each difference indicated was determined using a two-tailed Student's t test. *p < 0.01.
Regulation of the Rim101 Pathway by Changes in Glycerophospholipid Asymmetry
The results described above reveal that the Rim101 pathway is important for the expression of RSB1, so we investigated whether changes in lipid asymmetry activate the Rim101 pathway. The amount of cleaved Rim101 was slightly increased in the flop mutants (pdr5Δ and pdr5Δ yor1Δ) compared with wild-type cells, indicating that the Rim101 pathway is activated (Figure 5, A and B). Moreover, we observed more prominent processing of Rim101 in the flip mutants (lem3Δ and dnf1Δ dnf2Δ). Similar results were observed in the pH experiments at all pH levels tested (Figure 3F). Thus, a change in lipid asymmetry caused by the flip mutations activates the Rim101 pathway more strongly than changes by the flop mutations. Because induction levels of Rsb1-HA are similar between pdr5Δ cells and lem3Δ or dnf1Δ dnf2Δ cells, activation of Rim101 cannot be correlated with the Rsb1 induction (Figure 5, A and B). A similar inconsistency was observed between lem3Δ cells and lem3Δ pdr5Δ cells. Although much higher expression of Rsb1-HA was observed in the lem3Δ pdr5Δ cells compared with the lem3Δ cells, similar Rim101 processing was observed (Figure 5, A and B). These results suggest that pathways other than the Rim101 pathway, such as a Mot3/Mck1-related pathway, transduce the lipid asymmetry signal forward, resulting in the expression of Rsb1 in the flop mutants.
Figure 5.
The Rim101 pathway is activated by changes in lipid asymmetry. (A) Total proteins (1.25 μg) prepared from KCY662 (wild-type), KCY689 (pdr5Δ), KCY1011 (yor1Δ pdr5Δ), KCY692 (lem3Δ), KCY1029 (dnf1Δ dnf2Δ), and KCY696 (lem3Δ pdr5Δ) cells, each harboring the pFI1 (HA-RIM101) plasmid, were incubated with Endo H and separated by SDS-PAGE, followed by immunoblotting with an anti-HA or anti-Pgk1 antibody. (B) The intensities of the band for HA-Rim101 and HA-Rim101ΔC presented in A were quantified using Image J software (http://rsb.info.nih.gov/ij/) and are expressed as a percentage reflecting the level of HA-Rim101ΔC relative to sum of the level of the HA-Rim101 plus HA-Rim101ΔC. Values represent the means ± SD from the experiment shown in A and three other independent experiments. The statistical significance of each difference as compared with results from wild-type cells bearing pFI1 was determined using a two-tailed Student's t test. *p < 0.05.
Rsb1 Expression Is Repressed Downstream of Rim101 by Nrg1
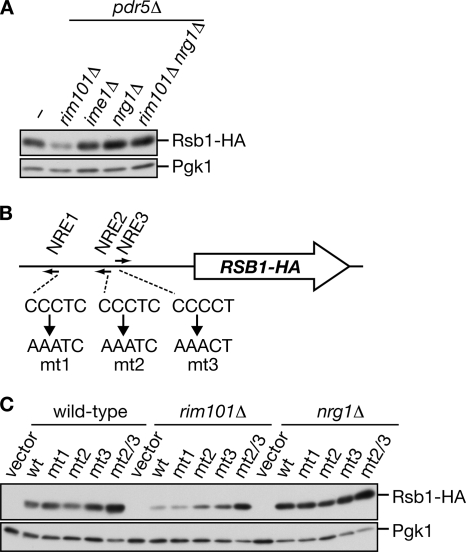
Because no Rim101 binding sequence exists in the putative promoter region of RSB1, it is unlikely that Rim101 directly regulates RSB1 transcription. Therefore, we searched for candidate transcription factors downstream of Rim101 using the Yeast search for transcriptional regulators and consensus tracking (YEASTRACT) database (http://www.yeastract.com). Of the potential transcription factors identified, two are known to be regulated by Rim101 at the transcriptional level, Ime1 (Su and Mitchell, 1993) and Nrg1 (Lamb and Mitchell, 2003), so we examined whether Rim101 regulates Rsb1 expression via one of these factors. Although an ime1Δ mutation had no effect on the pdr5Δ-caused Rsb1 expression, an nrg1Δ mutation further enhanced the expression (Figure 6A). Moreover, the nrg1Δ mutation bypassed the effect of the rim101Δ mutation, indicating that Nrg1 functions in Rsb1 induction downstream of Rim101. Rim101 reportedly functions as a repressor for the transcription of Nrg1, which normally represses the expression of pH-inducible genes (Lamb and Mitchell, 2003).
Figure 6.
RSB1 gene expression is repressed by Nrg1, downstream of Rim101. (A) KCY689 (pdr5Δ), KCY1016 (rim101Δ pdr5Δ), KCY1060 (ime1Δ pdr5Δ), KCY1061 (nrg1Δ pdr5Δ), and KCY1070 (rim101Δ nrg1Δ pdr5Δ) cells were grown in YPD medium. Total proteins (1.7 μg) prepared from each culture were incubated with Endo H, separated by SDS-PAGE, and subject to immunoblotting with an anti-HA or anti-Pgk1 antibody. (B) Schematic representation of the position and sequences of NREs. The locations of the 5′-nucleotides of NREs are at −761 (NRE1), −470 (NRE2), and −460 (NRE3) relative to the transcription start site. The sequences of the mt1-3 mutations are also shown. (C) The pRS423 (vector), pIKD412 (wild-type; wt), pIKD414 (mt1), pIKD416 (mt2), pIKD418 (mt3), and pIKD420 (mt2/3) plasmids were introduced into SEY6210 (wild-type), KCY595 (rim101Δ), and KCY594 (nrg1Δ) cells. Cells were precultured in SC medium lacking histidine, transferred to YPD medium, and grown to logarithmic phase. Total proteins (1.25 μg) prepared from each culture were treated with Endo H, separated by SDS-PAGE, and subject to immunoblotting with an anti-HA or anti-Pgk1 antibody.
RSB1 has three possible Nrg1-responsive elements (NREs 1-3) in its promoter region (Figure 6B). To determine which element might be important for Nrg1 binding, an RSB1-HA-expressing plasmid with its promoter region intact (wt) or carrying one or more mutated NRE sequence (Figure 6B) was introduced into wild-type, rim101Δ, and nrg1Δ cells. In nrg1Δ cells maximal expression occurred whether the promoter region was intact or mutated (Figure 6C), due to the absence of Nrg1, the repressor for RSB1-HA mRNA expression. In contrast, Rsb1-HA expression in wild-type cells was slightly increased by the mutation in NRE3 (mt3) and significantly increased by mutations in both NRE2 and NRE3 (mt2/3) compared with the Rsb1 level expressed from the intact promoter. These effects were more evident when the mutated plasmids were introduced into the rim101Δ cells (Figure 6C). Expression of Rsb1-HA from the mt2/3 construct in either wild-type cells or rim101Δ cells was similar to that observed in the nrg1Δ cells. These results suggest that both NRE2 and NRE3 are binding sites for Nrg1.
DISCUSSION
Of five genes identified in this study as coding for the presumed lipid asymmetry-sensing factor or a related protein, three (RIM13, RIM20, and RIM21) are part of the pH-responsive Rim101 pathway. Further analyses revealed that the Rim101 pathway itself is required for Rsb1 induction (Figure 3, C and D). In agreement with this result, a genome-wide microarray analysis found that the RSB1 gene was down-regulated by a rim101Δ or rim13Δ mutation (Lamb and Mitchell, 2003). The Rim101 pathway functions in pH adaptation. In S. cerevisiae, six RIM genes (RIM8, RIM9, RIM13, RIM20, RIM21, and RIM101), DFG16, YGR122w, and certain ESCRT genes are required for this pathway. The proposed signaling mechanism for this pathway includes several proteins homologous to other known signaling proteins. Dfg16 and Rim21 are homologues to PalH, an Aspergillus nidulans protein that interacts with the arrestin homolog PalF (Herranz et al., 2005). Because its multimembrane structure and its interaction with arrestin-like protein are characteristic features of receptor proteins, PalH is considered to be a pH sensor (Peñalva and Arst, 2004; Herranz et al., 2005). By homology, Dfg16 and/or Rim21 are also candidates for being pH sensors. Another multimembrane protein, Rim9, seems to function in association with Dfg16 and Rim21. Dfg16, Rim21, and Rim9 may act as pH sensor subunits or may be required for biogenesis of a sensor protein, e.g., Dfg16 or Rim21. Once Dfg16 or Rim21 recognizes the external alkaline pH, it is subjected to endocytosis by the assistance of the arrestin/PalF homolog Rim8. The adaptor protein Rim20 is then recruited to the endosomal compartment from the cytosol and associates with the ESCRT protein Snf7 (Boysen and Mitchell, 2006), although the molecular mechanism that links Dfg16/Rim21 and Rim20 is unclear. Snf7 seems to also interact with the protease Rim13 (Ito et al., 2001) and to form a multiprotein complex on the endosomal membrane. Rim20 recruits Rim101 to this complex, resulting in the processing of Rim101 by Rim13. The ESCRT-IIIB components are required for dissociation of this complex (Babst et al., 2002), so their mutations cause constitutive activation of Rim101 (Hayashi et al., 2005). The processed Rim101 enters the nucleus and represses the Rim101-responsive genes (Lamb and Mitchell, 2003).
Mutants of type 4 P-type ATPases or Cdc50 family members exhibit not only changes in membrane lipid asymmetry but also in vesicular trafficking functions such as post-Golgi transport and endocytosis (Graham, 2004). The possibility, then, that reduced vesicular transport activity or altered lipid composition indirectly induce Rsb1 expression cannot be excluded. However, none of the mutants affecting post-Golgi transport (vps45Δ), endocytosis (pcl1Δ and chc1Δ), or ergosterol biosynthesis (erg3Δ) induced Rsb1 expression (Supplementary Figure 1), suggesting that this possibility is unlikely. Therefore, it is most probable that changes in lipid asymmetry directly induce Rsb1 expression.
At present, it is unclear what the putative sensor proteins Rim21 and/or Dfg16 recognize. Several target molecules can be considered. For example, it is possible that they recognize cell wall components, because the Rim101 pathway is required for normal cell wall assembly (Castrejon et al., 2006). Inconsistent with this possibility, however, is our finding that cell wall mutants (krt6Δ and gas1Δ) did not induce Rsb1 expression (Supplementary Figure 1). We rather prefer the model, then, that the Rim101 pathway recognizes a cell surface charge. Under normal conditions, the negatively charged phospholipids PI, PS, and phosphatidic acid are confined to the inner leaflet of the plasma membrane. However, changes in lipid asymmetry can expose these negatively charged phospholipids on the cell surface. The putative sensor proteins Rim21 and/or Dfg16 may recognize the exposed negative charge similarly to the way they sense a charge in culture medium (hydroxyl ion or proton) under alkaline conditions.
Cooperation between the Rim101 pathway and Mck1 or Mot3 is known in the induction of other genes. For example, both the Rim101 pathway and Mck1 act independently to induce the transcription factor Ime1 (Su and Mitchell, 1993). In addition, the Rim101 pathway and Mot3 are linked via the Tup1-Cyc8 repression complex. Gene repression by Rim101 was shown to be dependent on Tup1-Cyc8 (Park et al., 1999; Lamb and Mitchell, 2003; Rothfels et al., 2005), and Mot3 is reportedly involved in the recruitment of Tup1-Cyc8 to its target sites (Klinkenberg et al., 2005). Therefore, the possibility cannot be excluded that links exist among the Rim101 pathway, Mck1, and Mot3. However, the interpretation that these pathways act independently on Rsb1 induction is more likely considering the additive effects of the rim101Δ mutation and the mck1Δ or mot3Δ mutation (Figure 4). In addition, neither the mot3Δ nor mck1Δ mutation affected the processing of Rim101 (data not shown).
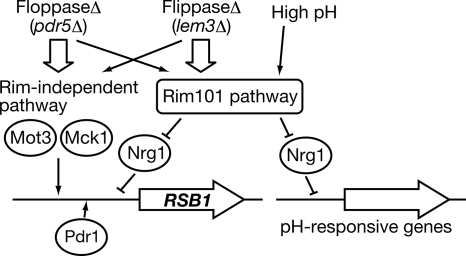
The lipid asymmetry signals induced by the flip mutation and by the flop mutation do not overlap completely. For example, the flip mutations (lem3Δ and dnf1Δ dnf2Δ) activated the Rim101 pathway more strongly than the flop mutation (pdr5Δ), although the Rsb1 induction levels were equivalent (Figure 5). Therefore, in the flop mutant other pathways, such as one involving Mot3/Mck1, must be activated more strongly than the Rim101 pathway (Figure 7).
Figure 7.
Model of the lipid asymmetry signaling pathway. A signal from moderate increases in pH induces expression of the pH-responsive genes through activation of the Rim101 pathway. The same Rim101 pathway transduces a signal resulting in Rsb1 expression, but other pathways, such as that involving Mot3 and Mck1, are also required. Changes in lipid asymmetry, caused either by a flip or flop mutation, induce activation of the Rim101 pathway and probably of the Mot3/Mck1-related pathways as well. However, these pathways are activated differently by the flip or flop mutation. The Rim101 pathway is activated more strongly by the flip mutation, whereas other pathways seem to be activated more predominantly by the flop mutation. Pdr1 may act as a basic transcription factor, since little Rsb1 expression was observed in pdr1Δ cells.
Although regulation of lipid asymmetry is important for several cellular functions and responses among eukaryotic cells, how changes in lipid asymmetry transduce a signal has been completely unknown. Thus, identification of a role for the Rim101 pathway in this study may provide an important clue for understanding other cellular events governed by lipid asymmetry.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. M. Snyder for providing the mTn-lacZ/LEU2-mutagenized yeast genomic library, Dr. T. Maeda for pFI1 plasmid, and Dr. M. Umeda for the Ro peptide. We are grateful to Dr. E. A. Sweeney for editing the manuscript. This study was supported by a Grant-in-Aid for Young Scientists (A) (17687011) from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan (A.K.) and by a grant from the Japan Society for the Promotion of Sciences (M.I.).
Abbreviations used:
- Endo H
endoglycosidase H
- ESCRT
endosomal sorting complex required for transport
- HA
hemagglutinin
- NRE
Nrg1-responsive element
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- Pgk1
phosphoglycerokinase 1
- PI
phosphatidylinositol
- PS
phosphatidylserine
- Ro
Ro 09-0198
- SC
synthetic complete
- SM
sphingomyelin.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-08-0806) on February 20, 2008.
REFERENCES
- Abramova N. E., Cohen B. D., Sertil O., Kapoor R., Davies K. J., Lowry C. V. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics. 2001;157:1169–1177. doi: 10.1093/genetics/157.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y., Uenaka T., Aoki J., Umeda M., Inoue K. A novel peptide probe for studying the transbilayer movement of phosphatidylethanolamine. J. Biochem. (Tokyo) 1994;116:291–297. doi: 10.1093/oxfordjournals.jbchem.a124522. [DOI] [PubMed] [Google Scholar]
- Babst M., Katzmann D. J., Estepa-Sabal E. J., Meerloo T., Emr S. D. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 2002;3:271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Bai J., Pagano R. E. Measurement of spontaneous transfer and transbilayer movement of BODIPY-labeled lipids in lipid vesicles. Biochemistry. 1997;36:8840–8848. doi: 10.1021/bi970145r. [DOI] [PubMed] [Google Scholar]
- Barwell K. J., Boysen J. H., Xu W., Mitchell A. P. Relationship of DFG16 to the Rim101p pH response pathway in Saccharomyces cerevisiae and Candida albicans. Eukaryot. Cell. 2005;4:890–899. doi: 10.1128/EC.4.5.890-899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boysen J. H., Mitchell A. P. Control of Bro1-domain protein Rim20 localization by external pH, ESCRT machinery, and the Saccharomyces cerevisiae Rim101 pathway. Mol. Biol. Cell. 2006;17:1344–1353. doi: 10.1091/mbc.E05-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns N., Grimwade B., Ross-Macdonald P. B., Choi E. Y., Finberg K., Roeder G. S., Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- Castrejon F., Gomez A., Sanz M., Duran A., Roncero C. The RIM101 pathway contributes to yeast cell wall assembly and its function becomes essential in the absence of mitogen-activated protein kinase Slt2p. Eukaryot. Cell. 2006;5:507–517. doi: 10.1128/EC.5.3.507-517.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton H. C., Ren B., Koh S. S., Harbison C. T., Kanin E., Jennings E. G., Lee T. I., True H. L., Lander E. S., Young R. A. Remodeling of yeast genome expression in response to environmental changes. Mol. Biol. Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Ingram M. F., Rosal P. H., Graham T. R. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 1999;147:1223–1236. doi: 10.1083/jcb.147.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Decottignies A., Grant A. M., Nichols J. W., de Wet H., McIntosh D. B., Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J. Biol. Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- Emoto K., Kobayashi T., Yamaji A., Aizawa H., Yahara I., Inoue K., Umeda M. Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis. Proc. Natl. Acad. Sci. USA. 1996;93:12867–12872. doi: 10.1073/pnas.93.23.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Fleming J. A., Lightcap E. S., Sadis S., Thoroddsen V., Bulawa C. E., Blackman R. K. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc. Natl. Acad. Sci. USA. 2002;99:1461–1466. doi: 10.1073/pnas.032516399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta N., Fujimura-Kamada K., Saito K., Yamamoto T., Tanaka K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell. 2007;18:295–312. doi: 10.1091/mbc.E06-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai E., Maeda T., Sorimachi H., Kitamoto K., Ishiura S., Suzuki K. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 1999;260:559–568. doi: 10.1007/s004380050929. [DOI] [PubMed] [Google Scholar]
- Gall W. E., Geething N. C., Hua Z., Ingram M. F., Liu K., Chen S. I., Graham T. R. Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 2002;12:1623–1627. doi: 10.1016/s0960-9822(02)01148-x. [DOI] [PubMed] [Google Scholar]
- Graham T. R. Flippases and vesicle-mediated protein transport. Trends Cell Biol. 2004;14:670–677. doi: 10.1016/j.tcb.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Hanson P. K., Malone L., Birchmore J. L., Nichols J. W. Lem3p is essential for the uptake and potency of alkylphosphocholine drugs, edelfosine and miltefosine. J. Biol. Chem. 2003;278:36041–36050. doi: 10.1074/jbc.M305263200. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Fukuzawa T., Sorimachi H., Maeda T. Constitutive activation of the pH-responsive Rim101 pathway in yeast mutants defective in late steps of the MVB/ESCRT pathway. Mol. Cell. Biol. 2005;25:9478–9490. doi: 10.1128/MCB.25.21.9478-9490.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz S., Rodriguez J. M., Bussink H. J., Sanchez-Ferrero J. C., Arst H. N., Jr, Peñalva M. A., Vincent O. Arrestin-related proteins mediate pH signaling in fungi. Proc. Natl. Acad. Sci. USA. 2005;102:12141–12146. doi: 10.1073/pnas.0504776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis J. C., Levine T. P. Lipid traffic: floppy drives and a superhighway. Nat. Rev. Mol. Cell. Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- Hongay C., Jia N., Bard M., Winston F. Mot3 is a transcriptional repressor of ergosterol biosynthetic genes and is required for normal vacuolar function in Saccharomyces cerevisiae. EMBO J. 2002;21:4114–4124. doi: 10.1093/emboj/cdf415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Z., Fatheddin P., Graham T. R. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell. 2002;13:3162–3177. doi: 10.1091/mbc.E02-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M., Kihara A., Igarashi Y. Lipid asymmetry of the eukaryotic plasma membrane: functions and related enzymes. Biol. Pharm. Bull. 2006;29:1542–1546. doi: 10.1248/bpb.29.1542. [DOI] [PubMed] [Google Scholar]
- Ito T., Chiba T., Ozawa R., Yoshida M., Hattori M., Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato U., Emoto K., Fredriksson C., Nakamura H., Ohta A., Kobayashi T., Murakami-Murofushi K., Umeda M. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J. Biol. Chem. 2002;277:37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- Katzmann D. J., Odorizzi G., Emr S. D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Kihara A., Igarashi Y. Identification and characterization of a Saccharomyces cerevisiae gene, RSB1, involved in sphingoid long-chain base release. J. Biol. Chem. 2002;277:30048–30054. doi: 10.1074/jbc.M203385200. [DOI] [PubMed] [Google Scholar]
- Kihara A., Igarashi Y. Cross talk between sphingolipids and glycerophospholipids in the establishment of plasma membrane asymmetry. Mol. Biol. Cell. 2004;15:4949–4959. doi: 10.1091/mbc.E04-06-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A., Igarashi Y. Synthesis, metabolism, and trans-bilayer movement of long-chain base. In: Hirabayashi Y., Igarashi Y., Merrill A. H. Jr, editors. Sphingolipid Biology. Tokyo: Springer; 2006. pp. 95–106. [Google Scholar]
- Klinkenberg L. G., Mennella T. A., Luetkenhaus K., Zitomer R. S. Combinatorial repression of the hypoxic genes of Saccharomyces cerevisiae by DNA binding proteins Rox1 and Mot3. Eukaryot. Cell. 2005;4:649–660. doi: 10.1128/EC.4.4.649-660.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. M., Mitchell A. P. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 2003;23:677–686. doi: 10.1128/MCB.23.2.677-686.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog. Lipid Res. 2003;42:423–438. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- Leonard P. J., Rathod P. K., Golin J. Loss of function mutation in the yeast multiple drug resistance gene PDR5 causes a reduction in chloramphenicol efflux. Antimicrob. Agents Chemother. 1994;38:2492–2494. doi: 10.1128/aac.38.10.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Mitchell A. P. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics. 1997;145:63–73. doi: 10.1093/genetics/145.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M. Y., Dailey D., Martin G. S., Thorner J. Yeast MCK1 protein kinase autophosphorylates at tyrosine and serine but phosphorylates exogenous substrates at serine and threonine. J. Biol. Chem. 1993;268:21155–21164. [PubMed] [Google Scholar]
- Manno S., Takakuwa Y., Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. USA. 2002;99:1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Máhe Y., Lemoine Y., Kuchler K. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J. Biol. Chem. 1996;271:25167–25172. doi: 10.1074/jbc.271.41.25167. [DOI] [PubMed] [Google Scholar]
- Natarajan P., Wang J., Hua Z., Graham T. R. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc. Natl. Acad. Sci. USA. 2004;101:10614–10619. doi: 10.1073/pnas.0404146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar S. L., Moye-Rowley W. S. Long chain base tolerance in Saccharomyces cerevisiae is induced by retrograde signals from the mitochondria. J. Biol. Chem. 2006;281:6376–6384. doi: 10.1074/jbc.M512115200. [DOI] [PubMed] [Google Scholar]
- Park S. H., Koh S. S., Chun J. H., Hwang H. J., Kang H. S. Nrg1 is a transcriptional repressor for glucose repression of STA1 gene expression in Saccharomyces cerevisiae. Mol. Cell. Biol. 1999;19:2044–2050. doi: 10.1128/mcb.19.3.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson J. K., Renkema K., Burden L., Halleck M. S., Schlegel R. A., Williamson P., Daleke D. L. Lipid specific activation of the murine P4-ATPase Atp8a1 (ATPase II) Biochemistry. 2006;45:5367–5376. doi: 10.1021/bi052359b. [DOI] [PubMed] [Google Scholar]
- Peñalva M. A., Arst H. N., Jr Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 2004;58:425–451. doi: 10.1146/annurev.micro.58.030603.123715. [DOI] [PubMed] [Google Scholar]
- Pomorski T., Holthuis J. C., Herrmann A., van Meer G. Tracking down lipid flippases and their biological functions. J. Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- Pomorski T., Lombardi R., Riezman H., Devaux P. F., van Meer G., Holthuis J. C. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell. 2003;14:1240–1254. doi: 10.1091/mbc.E02-08-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. S., Klionsky D. J., Banta L. M., Emr S. D. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfels K., Tanny J. C., Molnar E., Friesen H., Commisso C., Segall J. Components of the ESCRT pathway, DFG16, and YGR122w are required for Rim101 to act as a corepressor with Nrg1 at the negative regulatory element of the DIT1 gene of Saccharomyces cerevisiae. Mol. Cell. Biol. 2005;25:6772–6788. doi: 10.1128/MCB.25.15.6772-6788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Fujimura-Kamada K., Furuta N., Kato U., Umeda M., Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol. Biol. Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. A., Lis J. T. A germline transformation analysis reveals flexibility in the organization of heat shock consensus elements. Nucleic Acids Res. 1987;15:2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit J. J., et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- Su S. S., Mitchell A. P. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics. 1993;133:67–77. doi: 10.1093/genetics/133.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura S., Kihara A., Iwaki S., Inokuchi J., Igarashi Y. Regulation of the transport and protein levels of the inositol phosphorylceramide mannosyltransferases Csg1 and Csh1 by the Ca2+-binding protein Csg2. J. Biol. Chem. 2007;282:8613–8621. doi: 10.1074/jbc.M606649200. [DOI] [PubMed] [Google Scholar]
- Ujhazy P., Ortiz D., Misra S., Li S., Moseley J., Jones H., Arias I. M. Familial intrahepatic cholestasis 1: studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- Umeda M., Emoto K. Membrane phospholipid dynamics during cytokinesis: regulation of actin filament assembly by redistribution of membrane surface phospholipid. Chem. Phys. Lipids. 1999;101:81–91. doi: 10.1016/s0009-3084(99)00057-2. [DOI] [PubMed] [Google Scholar]
- van Helvoort A., Smith A. J., Sprong H., Fritzsche I., Schinkel A. H., Borst P., van Meer G. MDR1 P-glycoprotein is a lipid translocase of broad specificity, while MDR3 P-glycoprotein specifically translocates phosphatidylcholine. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Choung S. Y., Kobayashi T., Inoue K., Higashijima T., Miyazawa T. Complex formation of peptide antibiotic Ro09-0198 with lysophosphatidylethanolamine: 1H NMR analyses in dimethyl sulfoxide solution. Biochemistry. 1990;29:113–118. doi: 10.1021/bi00453a013. [DOI] [PubMed] [Google Scholar]
- Wang L., Beserra C., Garbers D. L. A novel aminophospholipid transporter exclusively expressed in spermatozoa is required for membrane lipid asymmetry and normal fertilization. Dev. Biol. 2004;267:203–215. doi: 10.1016/j.ydbio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Xu W., Mitchell A. P. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 2001;183:6917–6923. doi: 10.1128/JB.183.23.6917-6923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Smith F. J., Jr, Subaran R., Mitchell A. P. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell. 2004;15:5528–5537. doi: 10.1091/mbc.E04-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaal R. F., Comfurius P., Bevers E. M. Lipid-protein interactions in blood coagulation. Biochim. Biophys. Acta. 1998;1376:433–453. doi: 10.1016/s0304-4157(98)00018-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.