Abstract
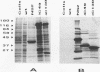
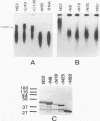
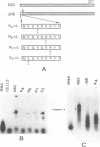
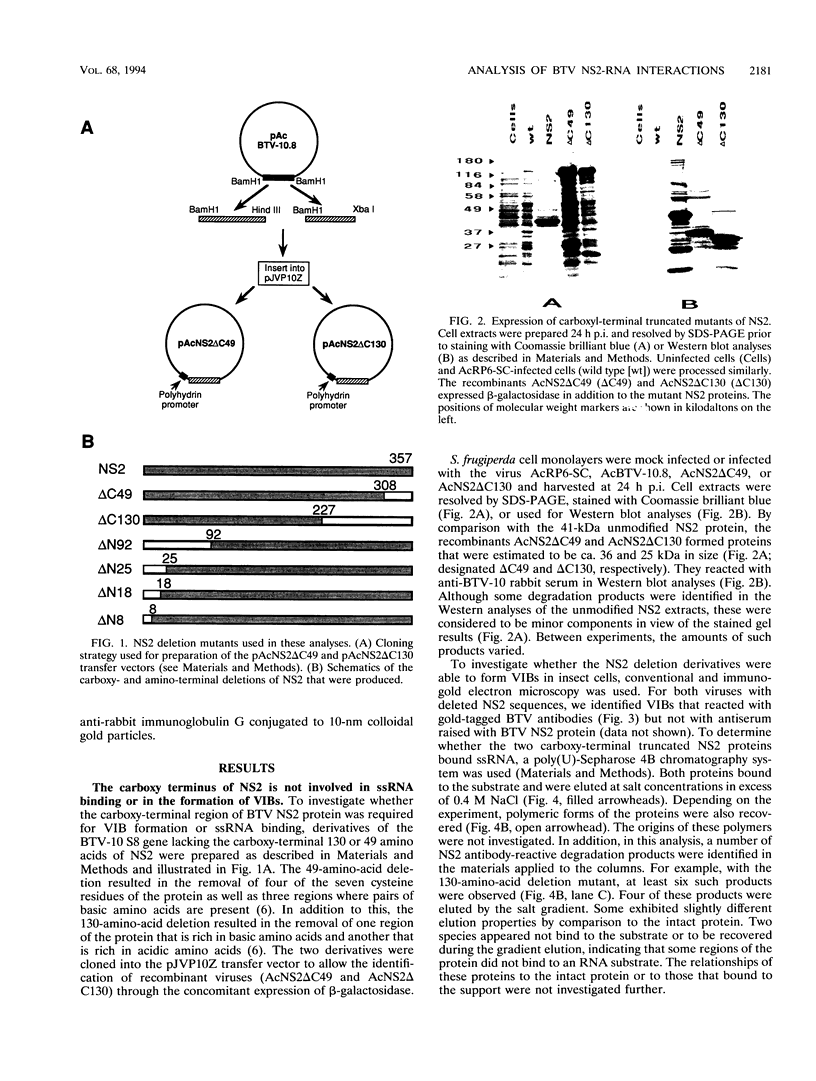
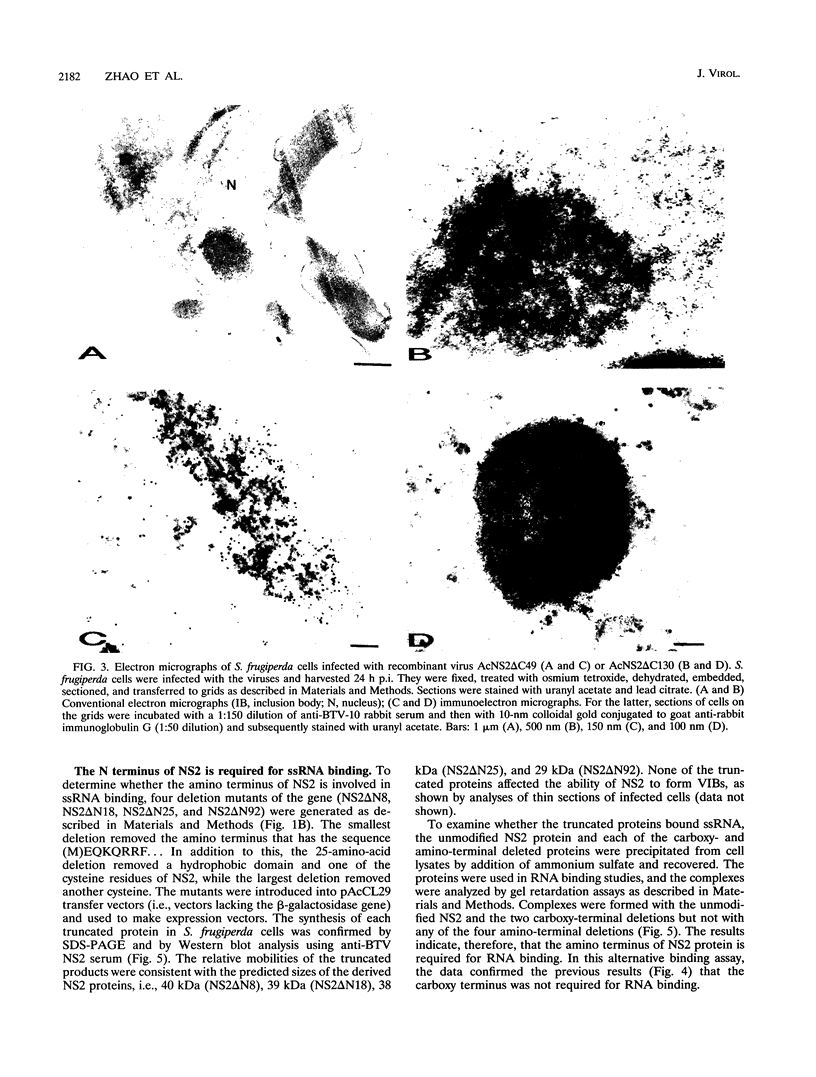
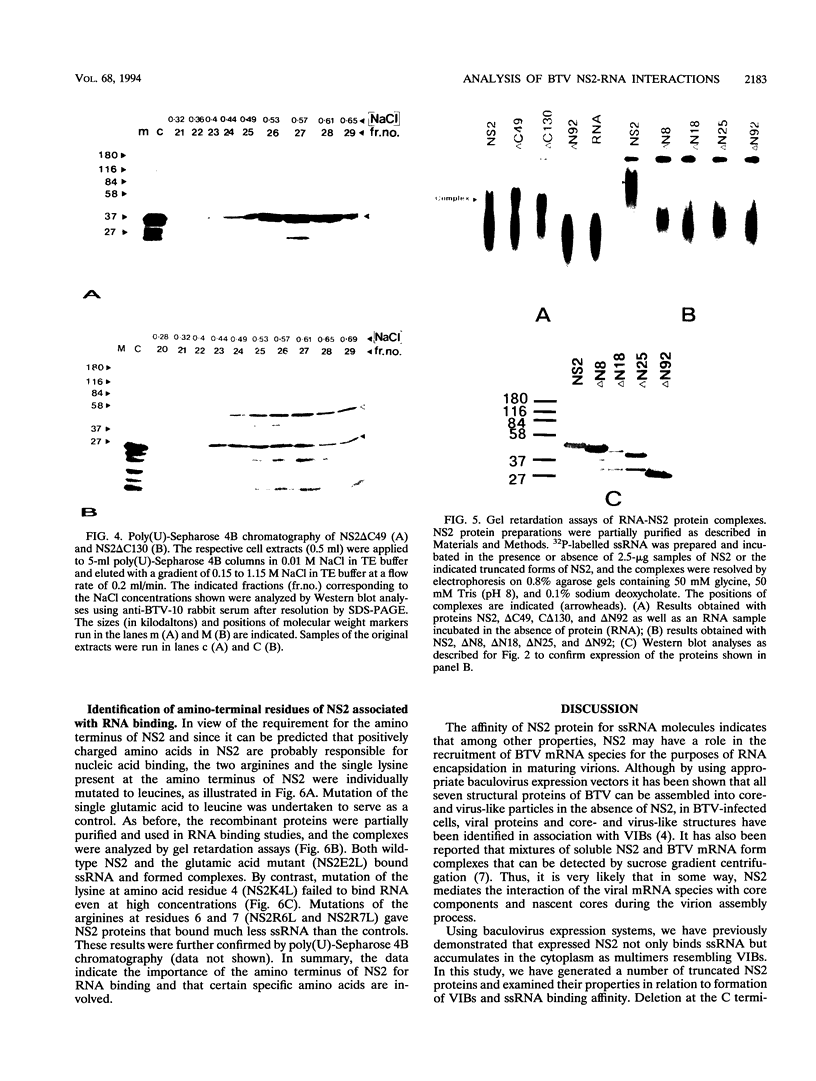
Genome segment 8 (S8) of bluetongue virus serotype 10 (BTV-10) encodes the nonstructural protein NS2. This protein, which has single-stranded RNA (ssRNA) binding capacity, is found in BTV-infected cells in the form of virus inclusion bodies (VIBs). To identify the domain(s) important for RNA binding and oligomerization of the protein, a number of deletions were made in regions of the gene that code for either the amino or carboxy terminus of the protein. The modified genes were cloned into and expressed from baculovirus vectors based on Autographa californica nuclear polyhedrosis virus. Truncated NS2 proteins were individually analyzed for the ability to bind ssRNA and to form VIBs. The results indicated that the carboxy terminus of the protein is involved neither in RNA binding nor in the formation of VIBs. The amino terminus of NS2 was shown to be essential for ssRNA binding but not for NS2 protein oligomerization. Point mutations that involved the substitution of various charged residues at the amino terminus of NS2 were generated and tested for the ability to bind ssRNA. The results showed that the arginines at amino acid residues 6 and 7 and the lysine at residue 4, but not the glutamic acid at residue 2, are involved in ssRNA binding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cordingley M. G., LaFemina R. L., Callahan P. L., Condra J. H., Sardana V. V., Graham D. J., Nguyen T. M., LeGrow K., Gotlib L., Schlabach A. J. Sequence-specific interaction of Tat protein and Tat peptides with the transactivation-responsive sequence element of human immunodeficiency virus type 1 in vitro. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8985–8989. doi: 10.1073/pnas.87.22.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. T., Hyatt A. D., Brookes S. M. The replication of bluetongue virus. Curr Top Microbiol Immunol. 1990;162:89–118. doi: 10.1007/978-3-642-75247-6_4. [DOI] [PubMed] [Google Scholar]
- French T. J., Inumaru S., Roy P. Expression of two related nonstructural proteins of bluetongue virus (BTV) type 10 in insect cells by a recombinant baculovirus: production of polyclonal ascitic fluid and characterization of the gene product in BTV-infected BHK cells. J Virol. 1989 Aug;63(8):3270–3278. doi: 10.1128/jvi.63.8.3270-3278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusho A., Yu Y., Yamaguchi S., Roy P. Completion of the sequence of bluetongue virus serotype 10 by the characterization of a structural protein, VP6, and a non-structural protein, NS2. J Gen Virol. 1989 Jul;70(Pt 7):1677–1689. doi: 10.1099/0022-1317-70-7-1677. [DOI] [PubMed] [Google Scholar]
- Huismans H., Van Dijk A. A. Bluetongue virus structural components. Curr Top Microbiol Immunol. 1990;162:21–41. doi: 10.1007/978-3-642-75247-6_2. [DOI] [PubMed] [Google Scholar]
- Huismans H., van Dijk A. A., Bauskin A. R. In vitro phosphorylation and purification of a nonstructural protein of bluetongue virus with affinity for single-stranded RNA. J Virol. 1987 Nov;61(11):3589–3595. doi: 10.1128/jvi.61.11.3589-3595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattoura M. D., Clapp L. L., Patton J. T. The rotavirus nonstructural protein, NS35, possesses RNA-binding activity in vitro and in vivo. Virology. 1992 Dec;191(2):698–708. doi: 10.1016/0042-6822(92)90245-k. [DOI] [PubMed] [Google Scholar]
- Kenan D. J., Query C. C., Keene J. D. RNA recognition: towards identifying determinants of specificity. Trends Biochem Sci. 1991 Jun;16(6):214–220. doi: 10.1016/0968-0004(91)90088-d. [DOI] [PubMed] [Google Scholar]
- Kitts P. A., Ayres M. D., Possee R. D. Linearization of baculovirus DNA enhances the recovery of recombinant virus expression vectors. Nucleic Acids Res. 1990 Oct 11;18(19):5667–5672. doi: 10.1093/nar/18.19.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Sharp P. A. Interactions between small nuclear ribonucleoprotein particles in formation of spliceosomes. Cell. 1987 Jun 19;49(6):763–774. doi: 10.1016/0092-8674(87)90614-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazinski D., Grzadzielska E., Das A. Sequence-specific recognition of RNA hairpins by bacteriophage antiterminators requires a conserved arginine-rich motif. Cell. 1989 Oct 6;59(1):207–218. doi: 10.1016/0092-8674(89)90882-9. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., Stone K. L., Cobianchi F., Wilson S. H., Williams K. R. Phenylalanines that are conserved among several RNA-binding proteins form part of a nucleic acid-binding pocket in the A1 heterogeneous nuclear ribonucleoprotein. J Biol Chem. 1988 Mar 5;263(7):3307–3313. [PubMed] [Google Scholar]
- Nagai K., Oubridge C., Jessen T. H., Li J., Evans P. R. Crystal structure of the RNA-binding domain of the U1 small nuclear ribonucleoprotein A. Nature. 1990 Dec 6;348(6301):515–520. doi: 10.1038/348515a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Söll D., Steitz T. A. Structure of E. coli glutaminyl-tRNA synthetase complexed with tRNA(Gln) and ATP at 2.8 A resolution. Science. 1989 Dec 1;246(4934):1135–1142. doi: 10.1126/science.2479982. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe J. W., Rhodes D. Beyond zinc fingers: steroid hormone receptors have a novel structural motif for DNA recognition. Trends Biochem Sci. 1991 Aug;16(8):291–296. doi: 10.1016/0968-0004(91)90121-b. [DOI] [PubMed] [Google Scholar]
- Thomas C. P., Booth T. F., Roy P. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J Gen Virol. 1990 Sep;71(Pt 9):2073–2083. doi: 10.1099/0022-1317-71-9-2073. [DOI] [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman G., Shoelson S. E., Pant N., Cowburn D., Kuriyan J. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell. 1993 Mar 12;72(5):779–790. doi: 10.1016/0092-8674(93)90405-f. [DOI] [PubMed] [Google Scholar]
- Wu X., Chen S. Y., Iwata H., Compans R. W., Roy P. Multiple glycoproteins synthesized by the smallest RNA segment (S10) of bluetongue virus. J Virol. 1992 Dec;66(12):7104–7112. doi: 10.1128/jvi.66.12.7104-7112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]