Abstract
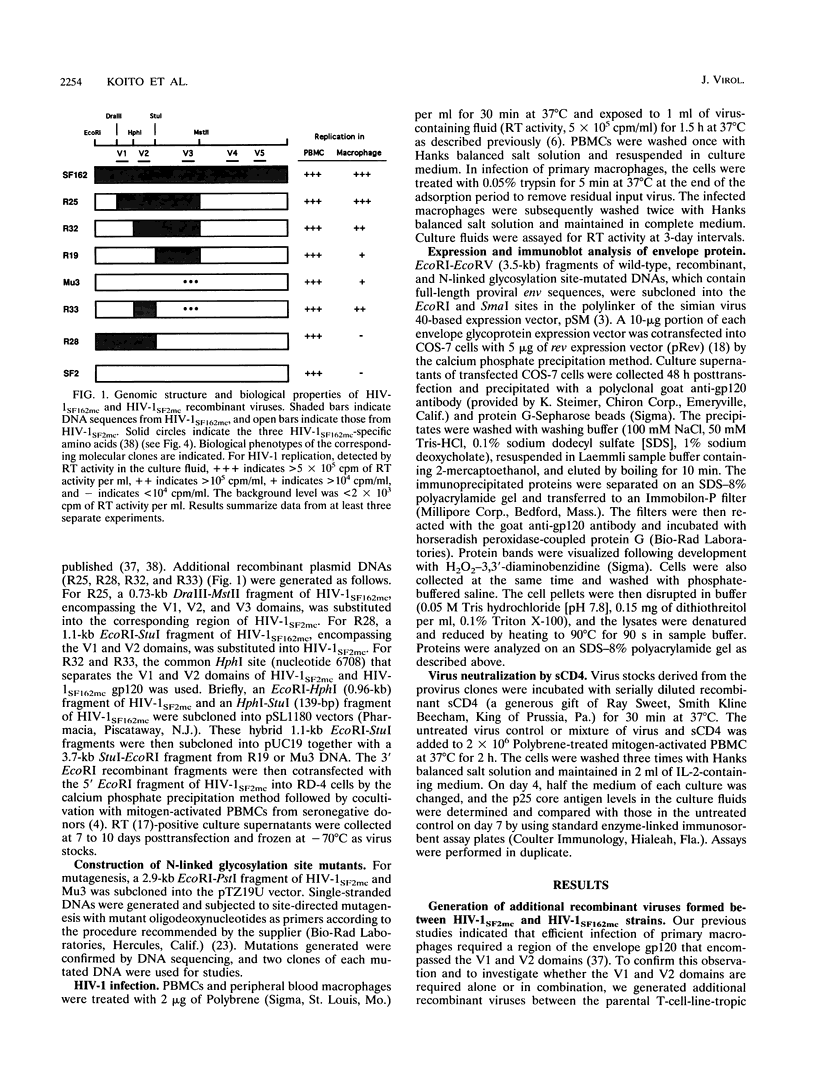
We have examined the influence of the V1/V2 region of the human immunodeficiency virus type 1 (HIV-1) gp120 on certain biologic properties of the virus. We observed that on the genomic background of the T-cell-line-tropic strain, HIV-1SF2mc, both the V1 and V2 domains of the macrophage-tropic strain, HIV-1SF162mc, in addition to the required V3 domain, are necessary to attain full macrophage tropism. Furthermore, the V2 domain modulates the sensitivity of HIV-1 to soluble CD4 neutralization. Structural studies of recombinant and mutant envelope glycoproteins suggest that the function of the V1/V2 region is to interact with the V3 domain and confer on the envelope gp120 of HIV-1SF2mc a conformation more similar to that of the macrophage-tropic strain HIV-1SF162mc. The conformation of the envelope gp120 appears to be strain specific and plays an important role in determining HIV-1 tissue tropism and sensitivity to soluble CD4 neutralization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andeweg A. C., Leeflang P., Osterhaus A. D., Bosch M. L. Both the V2 and V3 regions of the human immunodeficiency virus type 1 surface glycoprotein functionally interact with other envelope regions in syncytium formation. J Virol. 1993 Jun;67(6):3232–3239. doi: 10.1128/jvi.67.6.3232-3239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. T., Simpson G. R., Cann A. J., Johnson M. A., Weiss R. A. A single amino acid substitution in the V1 loop of human immunodeficiency virus type 1 gp120 alters cellular tropism. J Virol. 1993 Jun;67(6):3649–3652. doi: 10.1128/jvi.67.6.3649-3652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky M. H., Warton M., Myers R. M., Littman D. R. Analysis of the site in CD4 that binds to the HIV envelope glycoprotein. J Immunol. 1990 Apr 15;144(8):3078–3086. [PubMed] [Google Scholar]
- Cheng-Mayer C., Quiroga M., Tung J. W., Dina D., Levy J. A. Viral determinants of human immunodeficiency virus type 1 T-cell or macrophage tropism, cytopathogenicity, and CD4 antigen modulation. J Virol. 1990 Sep;64(9):4390–4398. doi: 10.1128/jvi.64.9.4390-4398.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Shioda T., Levy J. A. Host range, replicative, and cytopathic properties of human immunodeficiency virus type 1 are determined by very few amino acid changes in tat and gp120. J Virol. 1991 Dec;65(12):6931–6941. doi: 10.1128/jvi.65.12.6931-6941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng-Mayer C., Weiss C., Seto D., Levy J. A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell-tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992 Nov;66(11):6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements G. J., Price-Jones M. J., Stephens P. E., Sutton C., Schulz T. F., Clapham P. R., McKeating J. A., McClure M. O., Thomson S., Marsh M. The V3 loops of the HIV-1 and HIV-2 surface glycoproteins contain proteolytic cleavage sites: a possible function in viral fusion? AIDS Res Hum Retroviruses. 1991 Jan;7(1):3–16. doi: 10.1089/aid.1991.7.3. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebenbichler C., Westervelt P., Carrillo A., Henkel T., Johnson D., Ratner L. Structure-function relationships of the HIV-1 envelope V3 loop tropism determinant: evidence for two distinct conformations. AIDS. 1993 May;7(5):639–646. [PubMed] [Google Scholar]
- Freed E. O., Risser R. Identification of conserved residues in the human immunodeficiency virus type 1 principal neutralizing determinant that are involved in fusion. AIDS Res Hum Retroviruses. 1991 Oct;7(10):807–811. doi: 10.1089/aid.1991.7.807. [DOI] [PubMed] [Google Scholar]
- Fung M. S., Sun C. R., Gordon W. L., Liou R. S., Chang T. W., Sun W. N., Daar E. S., Ho D. D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992 Feb;66(2):848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaila R. J., Fuller B. A., Rennert P. D., Nelson M. B., Hammarskjöld M. L., Potts B., Murray M., Putney S. D., Gray G. Mutations in the principal neutralization determinant of human immunodeficiency virus type 1 affect syncytium formation, virus infectivity, growth kinetics, and neutralization. J Virol. 1992 Apr;66(4):1875–1883. doi: 10.1128/jvi.66.4.1875-1883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenink M., Fouchier R. A., Broersen S., Baker C. H., Koot M., van't Wout A. B., Huisman H. G., Miedema F., Tersmette M., Schuitemaker H. Relation of phenotype evolution of HIV-1 to envelope V2 configuration. Science. 1993 Jun 4;260(5113):1513–1516. doi: 10.1126/science.8502996. [DOI] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Fung M. S., Cao Y. Z., Li X. L., Sun C., Chang T. W., Sun N. C. Another discontinuous epitope on glycoprotein gp120 that is important in human immunodeficiency virus type 1 neutralization is identified by a monoclonal antibody. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8949–8952. doi: 10.1073/pnas.88.20.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Hope T. J., Huang X. J., McDonald D., Parslow T. G. Steroid-receptor fusion of the human immunodeficiency virus type 1 Rev transactivator: mapping cryptic functions of the arginine-rich motif. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7787–7791. doi: 10.1073/pnas.87.19.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of envelope V3 loop as the major determinant of CD4 neutralization sensitivity of HIV-1. Science. 1992 Jul 24;257(5069):535–537. doi: 10.1126/science.1636088. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Dubay J. W., Morris J. F., Roberts S. J., Gutshall L., Sternberg E. J., Hunter E., Matthews T. J., Petteway S. R., Jr V3 loop region of the HIV-1 gp120 envelope protein is essential for virus infectivity. Virology. 1992 Apr;187(2):423–432. doi: 10.1016/0042-6822(92)90444-t. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lee W. R., Syu W. J., Du B., Matsuda M., Tan S., Wolf A., Essex M., Lee T. H. Nonrandom distribution of gp120 N-linked glycosylation sites important for infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2213–2217. doi: 10.1073/pnas.89.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeating J. A., Shotton C., Cordell J., Graham S., Balfe P., Sullivan N., Charles M., Page M., Bolmstedt A., Olofsson S. Characterization of neutralizing monoclonal antibodies to linear and conformation-dependent epitopes within the first and second variable domains of human immunodeficiency virus type 1 gp120. J Virol. 1993 Aug;67(8):4932–4944. doi: 10.1128/jvi.67.8.4932-4944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Sattentau Q. J., Yoshiyama H., Thali M., Charles M., Sullivan N., Poon S. W., Fung M. S., Traincard F., Pinkus M. Probing the structure of the V2 domain of human immunodeficiency virus type 1 surface glycoprotein gp120 with a panel of eight monoclonal antibodies: human immune response to the V1 and V2 domains. J Virol. 1993 Oct;67(10):6136–6151. doi: 10.1128/jvi.67.10.6136-6151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Chen I. S., Ho D. D., Daar E. S. Mapping genetic determinants for human immunodeficiency virus type 1 resistance to soluble CD4. J Virol. 1992 May;66(5):3125–3130. doi: 10.1128/jvi.66.5.3125-3130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien W. A., Koyanagi Y., Namazie A., Zhao J. Q., Diagne A., Idler K., Zack J. A., Chen I. S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990 Nov 1;348(6296):69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Helseth E., Furman C., Li J., Haseltine W., Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990 Dec;64(12):5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orloff S. L., Kennedy M. S., Belperron A. A., Maddon P. J., McDougal J. S. Two mechanisms of soluble CD4 (sCD4)-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) infectivity and their relation to primary HIV-1 isolates with reduced sensitivity to sCD4. J Virol. 1993 Mar;67(3):1461–1471. doi: 10.1128/jvi.67.3.1461-1471.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page K. A., Stearns S. M., Littman D. R. Analysis of mutations in the V3 domain of gp160 that affect fusion and infectivity. J Virol. 1992 Jan;66(1):524–533. doi: 10.1128/jvi.66.1.524-533.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B. D., Poiesz B. J., Loeb L. A. Fidelity of HIV-1 reverse transcriptase. Science. 1988 Nov 25;242(4882):1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Moore J. P. Conformational changes induced in the human immunodeficiency virus envelope glycoprotein by soluble CD4 binding. J Exp Med. 1991 Aug 1;174(2):407–415. doi: 10.1084/jem.174.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Small amino acid changes in the V3 hypervariable region of gp120 can affect the T-cell-line and macrophage tropism of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9434–9438. doi: 10.1073/pnas.89.20.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N., Thali M., Furman C., Ho D. D., Sodroski J. Effect of amino acid changes in the V1/V2 region of the human immunodeficiency virus type 1 gp120 glycoprotein on subunit association, syncytium formation, and recognition by a neutralizing antibody. J Virol. 1993 Jun;67(6):3674–3679. doi: 10.1128/jvi.67.6.3674-3679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y., Akutsu M., Murayama K., Shimizu N., Hoshino H. Host range mutant of human immunodeficiency virus type 1: modification of cell tropism by a single point mutation at the neutralization epitope in the env gene. J Virol. 1991 Apr;65(4):1710–1718. doi: 10.1128/jvi.65.4.1710-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Levy J. A. Human immunodeficiency virus type 1 envelope gp120 is cleaved after incubation with recombinant soluble CD4. J Virol. 1993 May;67(5):2566–2574. doi: 10.1128/jvi.67.5.2566-2574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Gendelman H. E., Ratner L. Identification of a determinant within the human immunodeficiency virus 1 surface envelope glycoprotein critical for productive infection of primary monocytes. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3097–3101. doi: 10.1073/pnas.88.8.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]




