Abstract
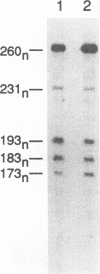
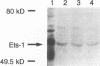
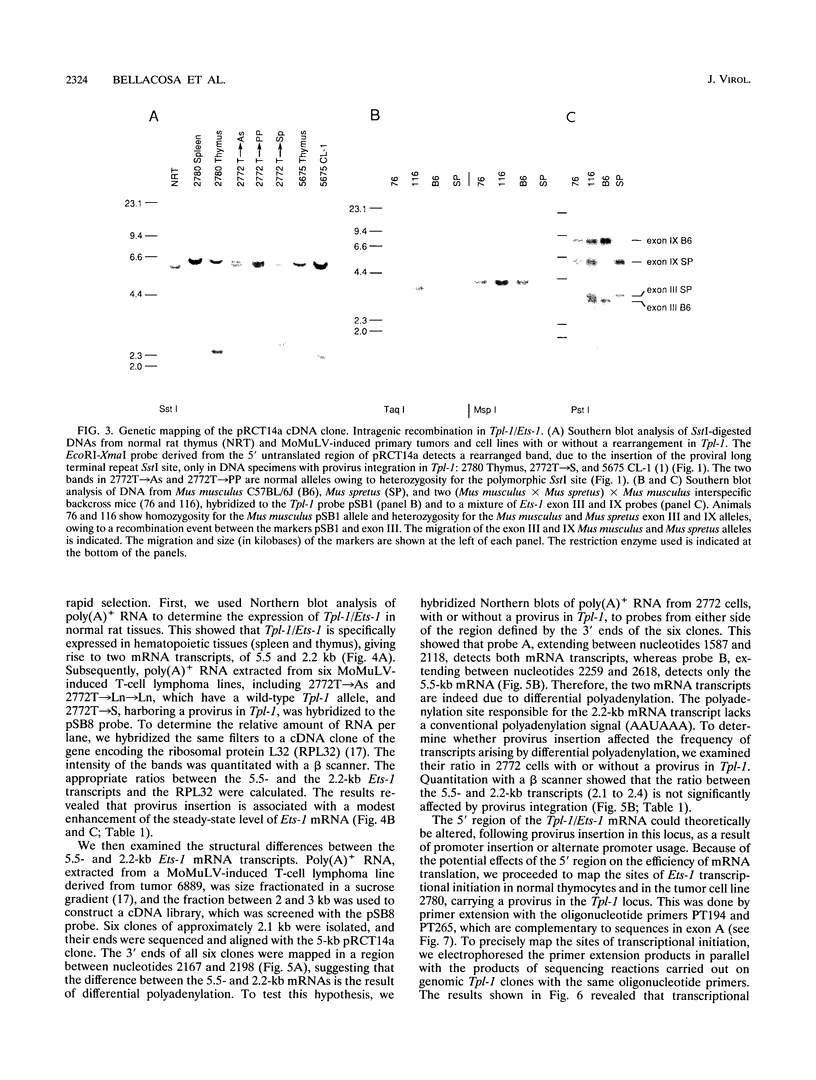
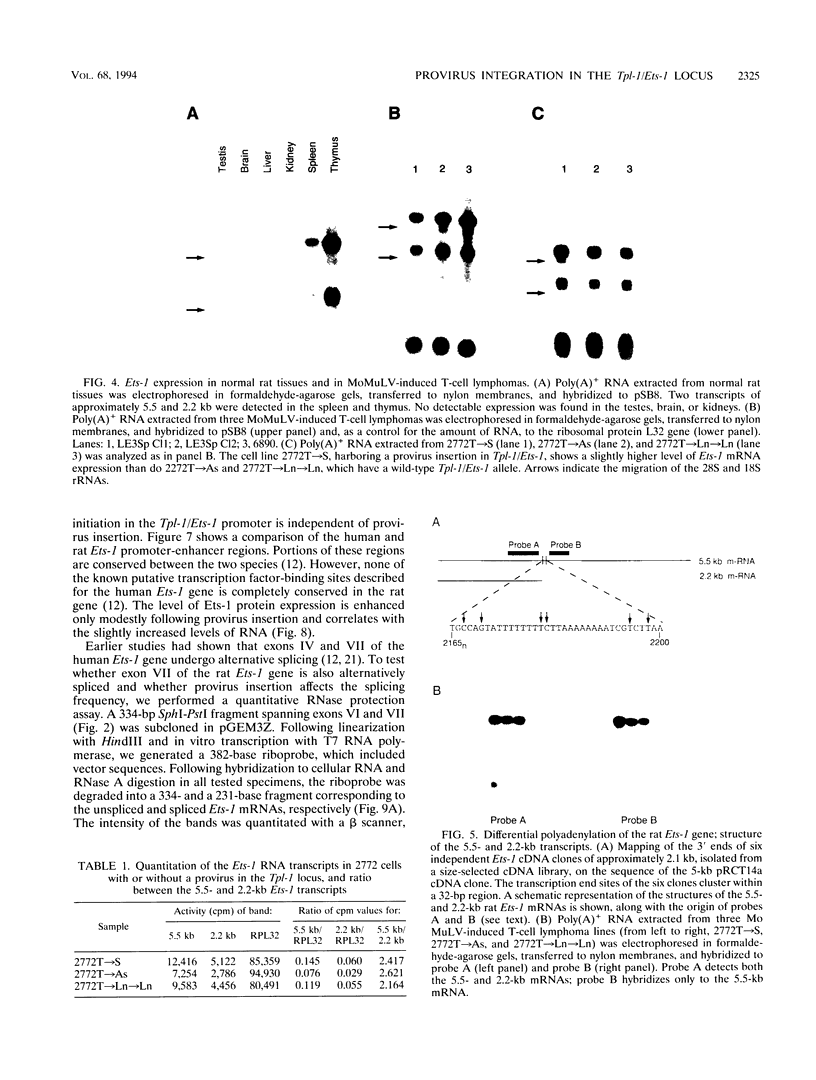
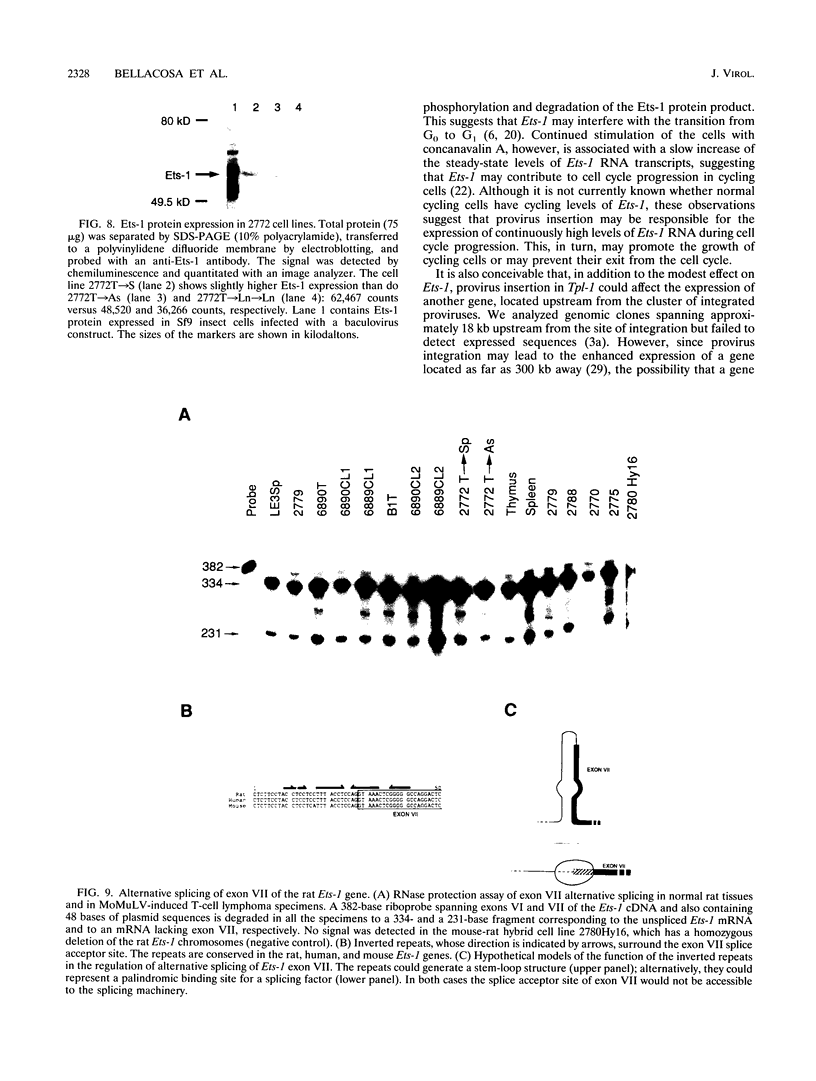
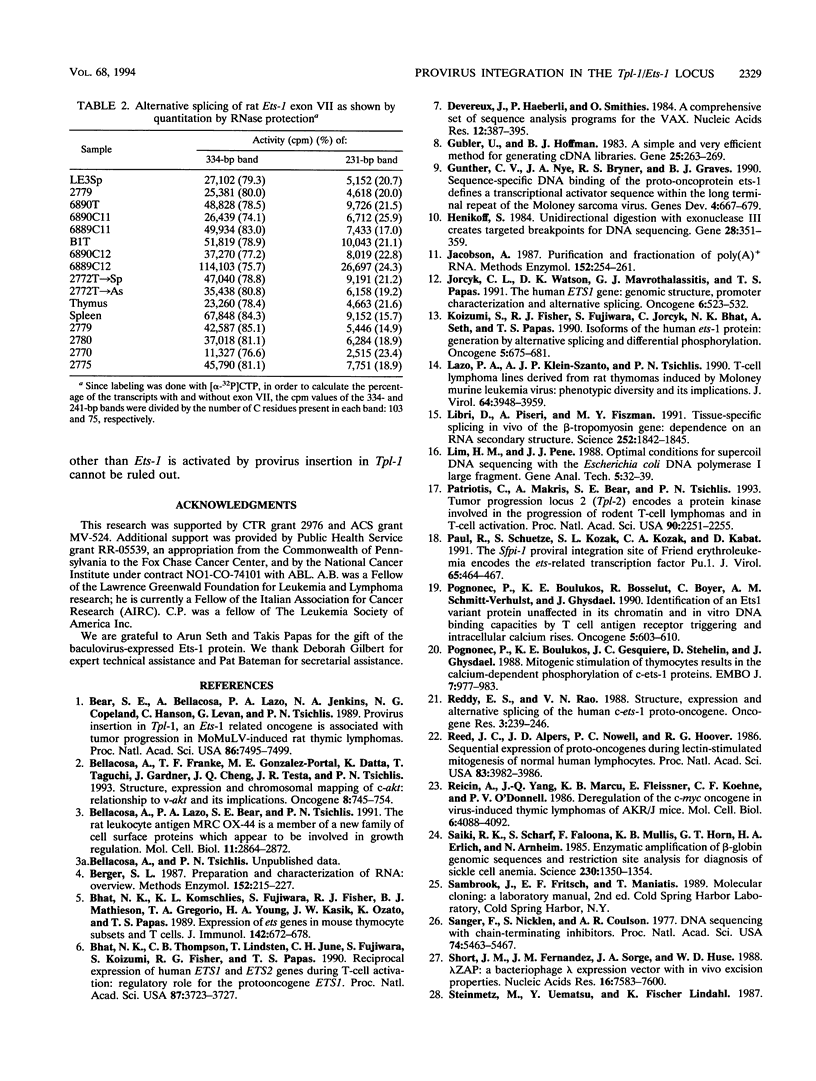
The Tpl-1 locus was defined as a genomic DNA region which is targeted by provirus insertion during progression of Moloney murine leukemia virus-induced rat T-cell lymphomas. Using a panel of 156 (Mus musculus x Mus spretus) x Mus musculus interspecific backcross mice, we mapped Tpl-1 to mouse chromosome 9 at a distance of 1.2 +/- 0.9 centimorgans from the Ets-1 proto-oncogene (S.E. Bear, A. Bellacosa, P.A. Lazo, N.A. Jenkins, N.G. Copeland, C. Hanson, G. Levan, and P.N. Tsichlis, Proc. Natl. Acad. Sci. USA 86:7495-7499, 1989). In this report, we present evidence that all the known Tpl-1 provirus insertions occurred immediately 5' of the first exon of Ets-1 (exon A) and that the earlier detected distance between Tpl-1 and Ets-1 was due to the high frequency of meiotic recombination in the region between the site of provirus integration and exon III. Northern (RNA) blot analysis of polyadenylated RNA from normal adult rat tissues and Moloney murine leukemia virus-induced T-cell lymphomas and hybridization to a Tpl-1/Ets-1 probe derived from the 5' end of the gene revealed two lymphoid cell-specific RNA transcripts, of 5.5 and 2.2 kb. Sequence analysis of a near-full-length (4,991-bp) cDNA clone of the 5.5-kb RNA revealed a 441-amino-acid open reading frame encoding a protein identical to the human and mouse Ets-1 proteins with the exception of five and nine species-specific conservative amino acid differences, respectively. The steady-state level of the Tpl-1/Ets-1 RNA and of the Ets-1 protein was modestly elevated in tumors carrying a provirus in the Tpl-1 locus. The relative ratio of the two Ets-1 transcripts, which were shown to arise by differential polyadenylation, was not affected by provirus insertion. Moreover, the major site of transcriptional initiation, which was localized by primer extension 250 bp upstream of the 5' end of the Ets-1 cDNA clone, was shown to be identical in normal cells and tumors carrying a provirus in the Tpl-1 locus. Finally, the differential splicing of Ets-1 exon VII was shown by RNase protection to occur at a rate of 15 to 26% and to remain unaffected by provirus insertion. The subtlety of these effects, in contrast to the strong growth selection of cells with a provirus in the Tpl-1/Ets-1 locus, suggests that provirus insertion may affect the fine regulation of the gene, perhaps during cell cycle progression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bear S. E., Bellacosa A., Lazo P. A., Jenkins N. A., Copeland N. G., Hanson C., Levan G., Tsichlis P. N. Provirus insertion in Tpl-1, an Ets-1-related oncogene, is associated with tumor progression in Moloney murine leukemia virus-induced rat thymic lymphomas. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7495–7499. doi: 10.1073/pnas.86.19.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellacosa A., Franke T. F., Gonzalez-Portal M. E., Datta K., Taguchi T., Gardner J., Cheng J. Q., Testa J. R., Tsichlis P. N. Structure, expression and chromosomal mapping of c-akt: relationship to v-akt and its implications. Oncogene. 1993 Mar;8(3):745–754. [PubMed] [Google Scholar]
- Bellacosa A., Lazo P. A., Bear S. E., Tsichlis P. N. The rat leukocyte antigen MRC OX-44 is a member of a new family of cell surface proteins which appear to be involved in growth regulation. Mol Cell Biol. 1991 May;11(5):2864–2872. doi: 10.1128/mcb.11.5.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L. Preparation and characterization of RNA: overview. Methods Enzymol. 1987;152:215–219. doi: 10.1016/0076-6879(87)52022-5. [DOI] [PubMed] [Google Scholar]
- Bhat N. K., Komschlies K. L., Fujiwara S., Fisher R. J., Mathieson B. J., Gregorio T. A., Young H. A., Kasik J. W., Ozato K., Papas T. S. Expression of ets genes in mouse thymocyte subsets and T cells. J Immunol. 1989 Jan 15;142(2):672–678. [PubMed] [Google Scholar]
- Bhat N. K., Thompson C. B., Lindsten T., June C. H., Fujiwara S., Koizumi S., Fisher R. J., Papas T. S. Reciprocal expression of human ETS1 and ETS2 genes during T-cell activation: regulatory role for the protooncogene ETS1. Proc Natl Acad Sci U S A. 1990 May;87(10):3723–3727. doi: 10.1073/pnas.87.10.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gunther C. V., Nye J. A., Bryner R. S., Graves B. J. Sequence-specific DNA binding of the proto-oncoprotein ets-1 defines a transcriptional activator sequence within the long terminal repeat of the Moloney murine sarcoma virus. Genes Dev. 1990 Apr;4(4):667–679. doi: 10.1101/gad.4.4.667. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Jacobson A. Purification and fractionation of poly(A)+ RNA. Methods Enzymol. 1987;152:254–261. doi: 10.1016/0076-6879(87)52028-6. [DOI] [PubMed] [Google Scholar]
- Jorcyk C. L., Watson D. K., Mavrothalassitis G. J., Papas T. S. The human ETS1 gene: genomic structure, promoter characterization and alternative splicing. Oncogene. 1991 Apr;6(4):523–532. [PubMed] [Google Scholar]
- Koizumi S., Fisher R. J., Fujiwara S., Jorcyk C., Bhat N. K., Seth A., Papas T. S. Isoforms of the human ets-1 protein: generation by alternative splicing and differential phosphorylation. Oncogene. 1990 May;5(5):675–681. [PubMed] [Google Scholar]
- Lazo P. A., Klein-Szanto A. J., Tsichlis P. N. T-cell lymphoma lines derived from rat thymomas induced by Moloney murine leukemia virus: phenotypic diversity and its implications. J Virol. 1990 Aug;64(8):3948–3959. doi: 10.1128/jvi.64.8.3948-3959.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libri D., Piseri A., Fiszman M. Y. Tissue-specific splicing in vivo of the beta-tropomyosin gene: dependence on an RNA secondary structure. Science. 1991 Jun 28;252(5014):1842–1845. doi: 10.1126/science.2063196. [DOI] [PubMed] [Google Scholar]
- Lim H. M., Pène J. J. Optimal conditions for supercoil DNA sequencing with the Escherichia coli DNA polymerase I large fragment. Gene Anal Tech. 1988 Mar-Apr;5(2):32–39. doi: 10.1016/0735-0651(88)90024-6. [DOI] [PubMed] [Google Scholar]
- Patriotis C., Makris A., Bear S. E., Tsichlis P. N. Tumor progression locus 2 (Tpl-2) encodes a protein kinase involved in the progression of rodent T-cell lymphomas and in T-cell activation. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2251–2255. doi: 10.1073/pnas.90.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Schuetze S., Kozak S. L., Kozak C. A., Kabat D. The Sfpi-1 proviral integration site of Friend erythroleukemia encodes the ets-related transcription factor Pu.1. J Virol. 1991 Jan;65(1):464–467. doi: 10.1128/jvi.65.1.464-467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Bosselut R., Boyer C., Schmitt-Verhulst A. M., Ghysdael J. Identification of a Ets1 variant protein unaffected in its chromatin and in vitro DNA binding capacities by T cell antigen receptor triggering and intracellular calcium rises. Oncogene. 1990 Apr;5(4):603–610. [PubMed] [Google Scholar]
- Pognonec P., Boulukos K. E., Gesquière J. C., Stéhelin D., Ghysdael J. Mitogenic stimulation of thymocytes results in the calcium-dependent phosphorylation of c-ets-1 proteins. EMBO J. 1988 Apr;7(4):977–983. doi: 10.1002/j.1460-2075.1988.tb02904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. S., Rao V. N. Structure, expression and alternative splicing of the human c-ets-1 proto-oncogene. Oncogene Res. 1988;3(3):239–246. [PubMed] [Google Scholar]
- Reed J. C., Alpers J. D., Nowell P. C., Hoover R. G. Sequential expression of protooncogenes during lectin-stimulated mitogenesis of normal human lymphocytes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3982–3986. doi: 10.1073/pnas.83.11.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reicin A., Yang J. Q., Marcu K. B., Fleissner E., Koehne C. F., O'Donnell P. V. Deregulation of the c-myc oncogene in virus-induced thymic lymphomas of AKR/J mice. Mol Cell Biol. 1986 Nov;6(11):4088–4092. doi: 10.1128/mcb.6.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Lazo P. A. Virus-host interactions and the pathogenesis of murine and human oncogenic retroviruses. Curr Top Microbiol Immunol. 1991;171:95–171. doi: 10.1007/978-3-642-76524-7_5. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Hu L. F. A common region for proviral DNA integration in MoMuLV-induced rat thymic lymphomas. 1983 Mar 31-Apr 6Nature. 302(5907):445–449. doi: 10.1038/302445a0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Strauss P. G., Lohse M. A. Concerted DNA rearrangements in Moloney murine leukemia virus-induced thymomas: a potential synergistic relationship in oncogenesis. J Virol. 1985 Oct;56(1):258–267. doi: 10.1128/jvi.56.1.258-267.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Lapis P., Lautenberger J. A., Schweinfest C. W., Papas T. S. Mammalian ets-1 and ets-2 genes encode highly conserved proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7862–7866. doi: 10.1073/pnas.85.21.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. K., McWilliams M. J., Papas T. S. Molecular organization of the chicken ets locus. Virology. 1988 May;164(1):99–105. doi: 10.1016/0042-6822(88)90624-1. [DOI] [PubMed] [Google Scholar]