Abstract
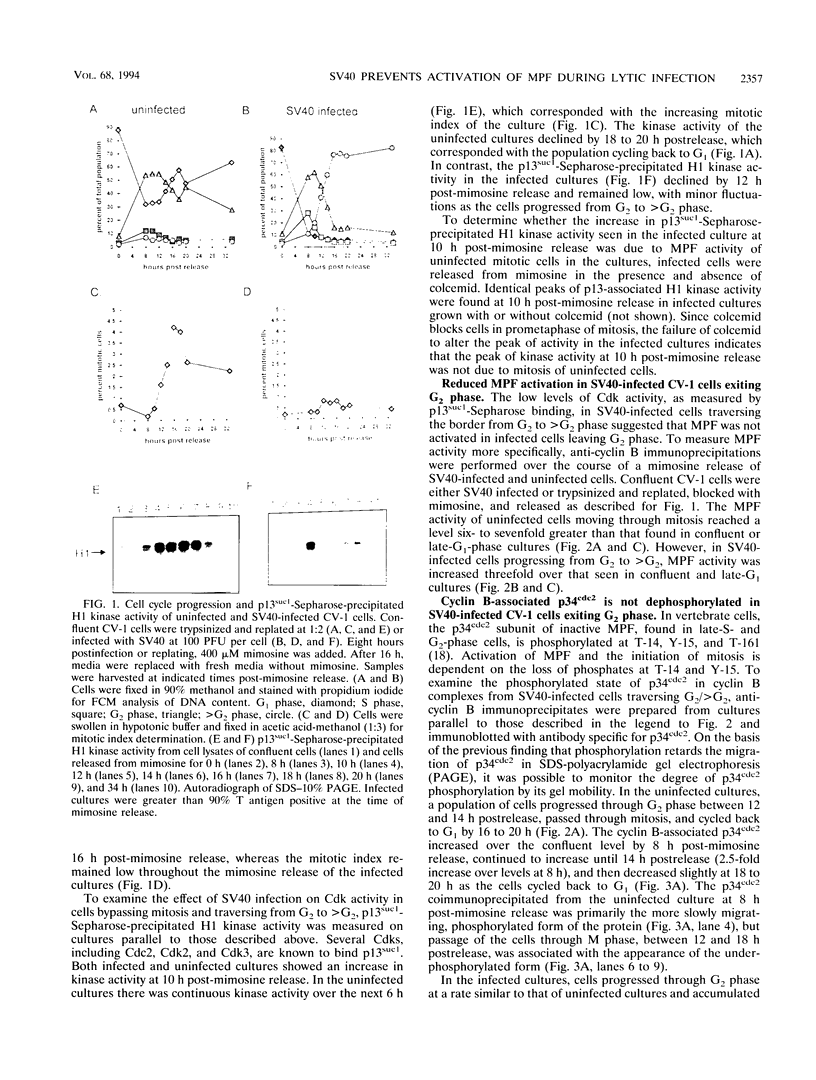
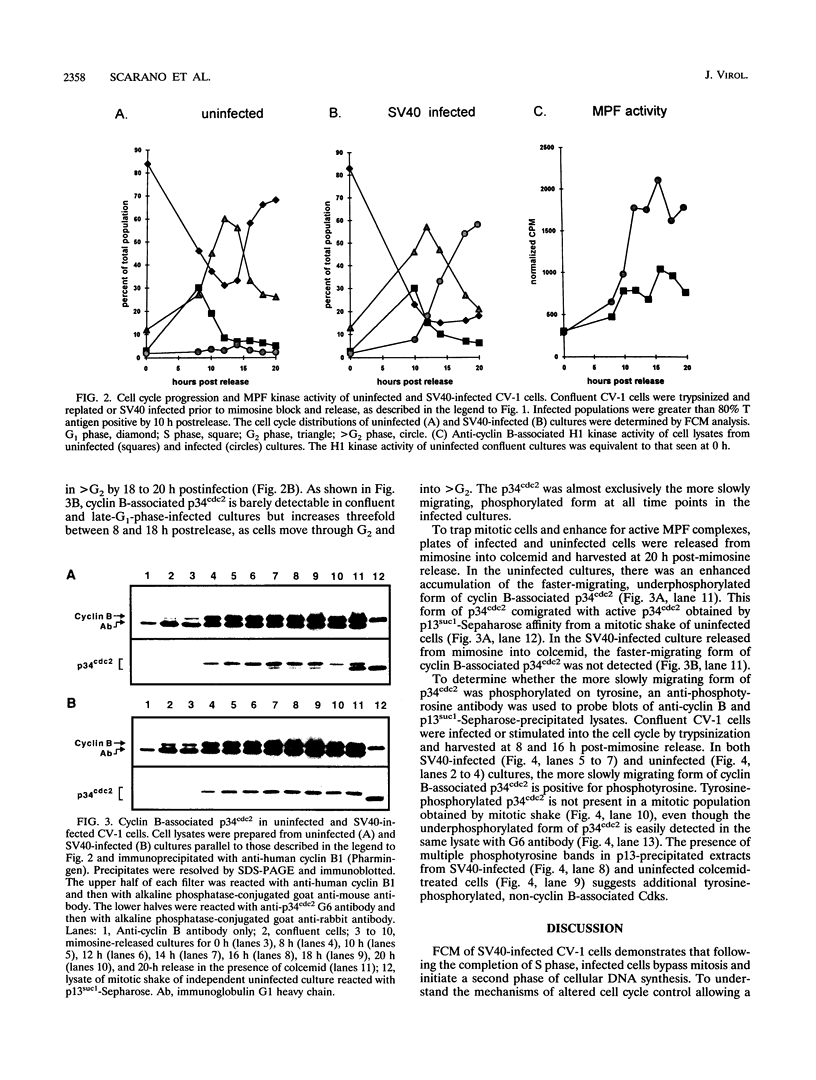
Simian virus 40 (SV40) infection stimulates confluent cultures of monkey kidney cells into successive rounds of cellular DNA synthesis without intervening mitosis. As an initial step in defining the mechanisms responsible for viral inhibition of mitosis, M-phase-promoting factor (MPF) was examined in SV40-infected CV-1 cells passing from G2 phase into a second S phase. MPF is a serine-threonine protein kinase that is essential for mitosis in eukaryotic cells. In SV40-infected cells exiting G2 phase, there was a reduced amount of MPF-associated H1 kinase activity relative to that of uninfected cells passing through mitosis. Both subunits of MPF, cyclin B and the p34cdc2 catalytic subunit, were present and in a complex in infected cells. In uninfected cultures, passage through mitosis was associated with the dephosphorylation of the p34cdc2 subunit, which is characteristic of MPF activation. In contrast, the p34cdc2 subunit remained in the tyrosine-phosphorylated, inactive form in SV40-infected cells passing from G2 phase into a second S phase. These results suggest that although the MPF complex is assembled and modified normally, SV40 interferes with pathways leading to MPF activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broek D., Bartlett R., Crawford K., Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991 Jan 31;349(6308):388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- Desai D., Gu Y., Morgan D. O. Activation of human cyclin-dependent kinases in vitro. Mol Biol Cell. 1992 May;3(5):571–582. doi: 10.1091/mbc.3.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelstein M., Arthur A. K., Dehde S., van Zee K., Dickmanns A., Fanning E. Intracistronic complementation reveals a new function of SV40 T antigen that co-operates with Rb and p53 binding to stimulate DNA synthesis in quiescent cells. Oncogene. 1992 May;7(5):837–847. [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Edgar B. A., O'Farrell P. H. Genetic control of cell division patterns in the Drosophila embryo. Cell. 1989 Apr 7;57(1):177–187. doi: 10.1016/0092-8674(89)90183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich T. D., Laffin J., Lehman J. M. Hypophosphorylated retinoblastoma gene product accumulates in SV40-infected CV-1 cells acquiring a tetraploid DNA content. Oncogene. 1993 Jun;8(6):1673–1677. [PubMed] [Google Scholar]
- Friedrich T. D., Laffin J., Lehman J. M. Simian virus 40 large T-antigen function is required for induction of tetraploid DNA content during lytic infection. J Virol. 1992 Jul;66(7):4576–4579. doi: 10.1128/jvi.66.7.4576-4579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaktionov K., Beach D. Specific activation of cdc25 tyrosine phosphatases by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991 Dec 20;67(6):1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- Gershey E. L. Simian virus 40-host cell interaction during lytic infection. J Virol. 1979 Apr;30(1):76–83. doi: 10.1128/jvi.30.1.76-83.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graessmann M., Graessman A. "Early" simian-virus-40-specific RNA contains information for tumor antigen formation and chromatin replication. Proc Natl Acad Sci U S A. 1976 Feb;73(2):366–370. doi: 10.1073/pnas.73.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaguchi J. R., Tobey R. A., Pines J., Crissman H. A., Hunter T., Bradbury E. M. Requirement for p34cdc2 kinase is restricted to mitosis in the mammalian cdc2 mutant FT210. J Cell Biol. 1992 Jun;117(5):1041–1053. doi: 10.1083/jcb.117.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R., McLoughlin M., McKeon F. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 1993 Aug 13;74(3):463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- Hoffmann I., Clarke P. R., Marcote M. J., Karsenti E., Draetta G. Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993 Jan;12(1):53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan P. K., Jett J. H., Romero A., Lehman J. M. Flow microfluorometry analysis of DNA content in Chinese hamster cells following infection with simian virus 40. Int J Cancer. 1974 Oct 15;14(4):514–521. doi: 10.1002/ijc.2910140411. [DOI] [PubMed] [Google Scholar]
- Jimenez J., Alphey L., Nurse P., Glover D. M. Complementation of fission yeast cdc2ts and cdc25ts mutants identifies two cell cycle genes from Drosophila: a cdc2 homologue and string. EMBO J. 1990 Nov;9(11):3565–3571. doi: 10.1002/j.1460-2075.1990.tb07567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Differential phosphorylation of vertebrate p34cdc2 kinase at the G1/S and G2/M transitions of the cell cycle: identification of major phosphorylation sites. EMBO J. 1991 Feb;10(2):305–316. doi: 10.1002/j.1460-2075.1991.tb07951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W., Nigg E. A. Mutations of p34cdc2 phosphorylation sites induce premature mitotic events in HeLa cells: evidence for a double block to p34cdc2 kinase activation in vertebrates. EMBO J. 1991 Nov;10(11):3331–3341. doi: 10.1002/j.1460-2075.1991.tb04897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffin J., Fogleman D., Lehman J. M. Correlation of DNA content, p53, T antigen, and V antigen in simian virus 40-infected human diploid cells. Cytometry. 1989 Mar;10(2):205–213. doi: 10.1002/cyto.990100212. [DOI] [PubMed] [Google Scholar]
- Laffin J., Lehman J. M. Detection of intracellular virus and viral products. Methods Cell Biol. 1990;33:271–284. doi: 10.1016/s0091-679x(08)60531-2. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Ogg S., Xu M., Parker L. L., Donoghue D. J., Maller J. L., Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992 Jan;3(1):73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman J. M., Bloustein P. Chromosome analysis and agglutination by concanavalin a of primary simian-virus-40-induced tumors. Int J Cancer. 1974 Dec 15;14(6):771–778. doi: 10.1002/ijc.2910140611. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Friedrich T. D., Laffin J. Quantitation of simian virus 40 T-antigen correlated with the cell cycle of permissive and non-permissive cells. Cytometry. 1993;14(4):401–410. doi: 10.1002/cyto.990140409. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Laffin J., Jacobberger J. W., Fogleman D. Analysis of simian virus 40 infection of CV-1 cells by quantitative two-color fluorescence with flow cytometry. Cytometry. 1988 Jan;9(1):52–59. doi: 10.1002/cyto.990090109. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Deshaies R. J. Exercising self-restraint: discouraging illicit acts of S and M in eukaryotes. Cell. 1993 Jul 30;74(2):223–226. doi: 10.1016/0092-8674(93)90413-k. [DOI] [PubMed] [Google Scholar]
- Lock R. B. Inhibition of p34cdc2 kinase activation, p34cdc2 tyrosine dephosphorylation, and mitotic progression in Chinese hamster ovary cells exposed to etoposide. Cancer Res. 1992 Apr 1;52(7):1817–1822. [PubMed] [Google Scholar]
- McGowan C. H., Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993 Jan;12(1):75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead P. S., Saksela E. The sequence of chromosome aberrations during SV 40 transformation of a human diploid cell strain. Hereditas. 1965;52(3):271–284. doi: 10.1111/j.1601-5223.1965.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Nigg E. A. Targets of cyclin-dependent protein kinases. Curr Opin Cell Biol. 1993 Apr;5(2):187–193. doi: 10.1016/0955-0674(93)90101-u. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor P. M., Ferris D. K., Pagano M., Draetta G., Pines J., Hunter T., Longo D. L., Kohn K. W. G2 delay induced by nitrogen mustard in human cells affects cyclin A/cdk2 and cyclin B1/cdc2-kinase complexes differently. J Biol Chem. 1993 Apr 15;268(11):8298–8308. [PubMed] [Google Scholar]
- Ornitz D. M., Hammer R. E., Messing A., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987 Oct 9;238(4824):188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992 Sep 25;257(5078):1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray F. A., Meyne J., Kraemer P. M. SV40 T antigen induced chromosomal changes reflect a process that is both clastogenic and aneuploidogenic and is ongoing throughout neoplastic progression of human fibroblasts. Mutat Res. 1992 Dec 16;284(2):265–273. doi: 10.1016/0027-5107(92)90011-p. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rinehart C. A., Mayben J. P., Butler T. D., Haskill J. S., Kaufman D. G. Alterations of DNA content in human endometrial stromal cells transfected with a temperature-sensitive SV40: tetraploidization and physiological consequences. Carcinogenesis. 1992 Jan;13(1):63–68. doi: 10.1093/carcin/13.1.63. [DOI] [PubMed] [Google Scholar]
- Sebastian B., Kakizuka A., Hunter T. Cdc25M2 activation of cyclin-dependent kinases by dephosphorylation of threonine-14 and tyrosine-15. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3521–3524. doi: 10.1073/pnas.90.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. V., Orr-Weaver T. L. The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development. 1991 Aug;112(4):997–1008. doi: 10.1242/dev.112.4.997. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Lee T., Kirschner M. W. Role of phosphorylation in p34cdc2 activation: identification of an activating kinase. Mol Biol Cell. 1992 Jan;3(1):13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Galanti N., Jonak G. J., McKercher S., Pipas J. M., Peden K. W., Baserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann K. E., Belinsky G. S., Lee D., Schlegel R. Chemically induced premature mitosis: differential response in rodent and human cells and the relationship to cyclin B synthesis and p34cdc2/cyclin B complex formation. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6843–6847. doi: 10.1073/pnas.88.15.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Th'ng J. P., Wright P. S., Hamaguchi J., Lee M. G., Norbury C. J., Nurse P., Bradbury E. M. The FT210 cell line is a mouse G2 phase mutant with a temperature-sensitive CDC2 gene product. Cell. 1990 Oct 19;63(2):313–324. doi: 10.1016/0092-8674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. A., Hanauske-Abel H. H., Flint A., Lalande M. Mimosine reversibly arrests cell cycle progression at the G1-S phase border. Cytometry. 1991;12(3):242–246. doi: 10.1002/cyto.990120306. [DOI] [PubMed] [Google Scholar]