Abstract
Phogat S, Wyatt RT, Karlsson Hedestam GB (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA; Department of Microbiology Tumor and Cell Biology, Karolinska Institutet, Stockholm; and the Swedish Institute for Infectious Disease Control, Solna, Sweden). Inhibition of HIV-1 entry by antibodies: potential viral and cellular targets (Review).
Vaccine-induced antibodies that interfere with viral entry are the protective correlate of most existing prophylactic vaccines. However, for highly variable viruses such as HIV-1, the ability to elicit broadly neutralizing antibody responses through vaccination has proven to be extremely difficult. The major targets for HIV-1 neutralizing antibodies are the viral envelope glycoprotein trimers on the surface of the virus that mediate receptor binding and entry. HIV-1 has evolved many mechanisms on the surface of envelope glyco-proteins to evade antibody-mediated neutralization, including the masking of conserved regions by glycan, quaternary protein interactions and the presence of immunodominant variable elements. The primary challenge in the development of an HIV-1 vaccine that elicits broadly neutralizing antibodies therefore lies in the design of suitable envelope glycoprotein immunogens that circumvent these barriers. Here, we describe neutralizing determinants on the viral envelope glyco-proteins that are defined by their function in receptor binding or by rare neutralizing antibodies isolated from HIV-infected individuals. We also describe the nonvariable cellular receptors involved in the HIV-1 entry process, or other cellular proteins, and ongoing studies to determine if antibodies against these proteins have efficacy as therapeutic reagents or, in some cases, as vaccine targets to interfere with HIV-1 entry.
Keywords: HIV-1, neutralizing antibodies, vaccine, envelope glycoprotein, receptors, incorporated proteins
Introduction
As the HIV-1 epidemic continuous unabated there is an urgent need to develop an effective prophylactic vaccine and other therapeutic strategies to limit viral transmission. Only a fraction of the nearly 40 million individuals living with the virus today receive the costly antiviral therapy currently available and these individuals are almost exclusively inhabitants of developed countries. It is therefore critical that preventative measures are taken to curb the infection rate in areas of the world harbouring the greatest HIV-1 burden. The most attractive strategies to deal with the HIV problem are the development of a preventative vaccine and the development of readily available effective microbicides. Both approaches are important and ultimately a combination of the two may prove the most effective in controlling the HIV-1 epidemic by decreasing the frequency of human-to-human transmission events.
The development of a protective vaccine against HIV-1 has proven to be an extremely challenging task but is under intensive pursuit, as a vaccine is the most cost-effective means to diminish the spread of the virus to uninfected individuals. Extensive research in this area suggests that a successful HIV-1 vaccine will need to stimulate both broadly neutralizing antibodies (Nabs) and potent HIV-1-specific T-cell responses. The importance of humoral immune responses has received renewed attention as it was shown that passive therapy with broadly neutralizing monoclonal antibodies against the HIV-1 surface proteins was protective in monkey challenge models [1–6]. An important component of any HIV-1 vaccine approach is therefore the viral envelope glycoproteins (Env), the major target for NAbs. There is an increasing understanding in the field that conserved structures of Env need to be targeted to obtain cross-neutralization between diverse virus strains and to minimize the likelihood of immunological escape. It is therefore crucial to define all detectable Env-directed neutralization specificities and to understand the molecular basis of HIV-1 neutralization resistance. Such studies, together with the development of new adjuvants and a better delineation of innate-to-adaptive immune signalling pathways, will hopefully provide the field with the requisite knowledge to approach the problem with better outcomes than observed to date.
For individuals already infected with HIV-1, highly active antiretroviral therapy (HAART) exists thanks to the extensive development over the past two decades of small molecular weight compounds targeting key viral processes, such as reverse transcription or protease-dependent Gag-Pol precursor protein cleavage (the major targets of HAART). A peptide-based inhibitor of the HIV-1 entry process, T-20, has gained acceptance as salvage therapy for individuals that harbour HAART escape mutants or that cannot tolerate HAART [7]. In addition, drugs capable of interfering with HIV-1 integration into the host genome [8] or virus binding to the coreceptors CCR5 or CXCR4 are currently in clinical trials [9] and therapeutic antibodies against the cellular targets CD4 (the primary HIV-1 receptor) and the major coreceptor, CCR5, have shown early promise [10–13]. Because a functional CCR5 allele is dispensable for survival in the developed world and because of its cell type-specific expression and its important role as the major core-ceptor for HIV-1, CCR5 has evolved as an attractive target for therapeutic intervention. Early promise of CCR5 as a therapeutic antibody target has lead to the consideration that CCR5 could be a vaccine target, but there are some distinct differences between a therapeutic antibody and an in vivo elicited antibody response against a self-molecule (see below).
Accordingly, in this review, we provide background information of the HIV-1 envelope glycoproteins that comprise the functional spike and discuss the properties, often elucidated by recent structures, which make the spike exceptionally challenging to target with NAbs. We then describe current strategies under pursuit to develop more effective HIV-1 Env vaccine immunogens and we review selected approaches to target cellular molecules as a means of inhibiting HIV-1 entry with therapeutic antibodies or vaccination.
The HIV-1 envelope glycoproteins and neutralizing determinants
The HIV-1 envelope glycoproteins and viral entry
HIV-1 is a member of the Retroviridae family belonging to the genus lentiviruses. The Retroviridae are enveloped viruses containing two positive sense RNA strands that are converted into dsDNA by the highly error-prone viral reverse transcriptase enzyme generating isolate diversity by both point mutation and intergenomic recombination. HIV-1 isolates fall into three groups: M (Major/Main), N (Non-M, Non-O/New) and O (Outlier) of which, as implied, group M is most common. Group M is subdivided into several subtypes or clades (A–D, F–H, J and K), of which B is most common in the Western world, whilst C is the predominant subtype found primarily in India, China and sub-Saharan Africa. The remaining subtypes, as well as HIV-1 variants with characteristics of several different subtypes, so-called circulating recombinant forms (CRFs), are dispersed throughout Africa and other parts of the world.
The major targets for HIV-1 NAbs are the exterior envelope glycoprotein, gp120, and the transmembrane glycoprotein, gp41. These proteins are generated by cleavage of a heavily glycosylated precursor protein, gp160, by furin-like enzymes during transport through the Golgi apparatus. Once transported to the cell surface, trimeric gp120/gp41 envelope glycoprotein spikes are incorporated into budding virus for release of new HIV-1 particles. Each new infectious cycle is initiated when the external envelope glycoprotein gp120 binds the primary receptor, CD4, which is embedded in the plasma membrane on the surface of potential targets cells (Fig. 1). Interaction of gp120 with CD4 is followed by a series of conformational changes in Env resulting in exposure of a transient binding site that allows the spike to interact with its coreceptor, usually CCR5 or CXCR4. This in turn promotes additional conformational changes that allow gp41 to insert its fusion peptide into the target cell membrane to form a prehairpin structure, which then collapses into an energetically stable six-helix bundle structure, driving virus-to-cell membrane fusion and entry of the HIV-1 core into the target cell [14]. This sequence of event occurs at the plasma membrane at neutral pH. Main target cells for HIV-1 infection are CD4+ T cells, macrophages and different subsets of dendritic cells (DC), whose relative roles during natural HIV-1 transmission remain poorly defined.
Fig. 1.
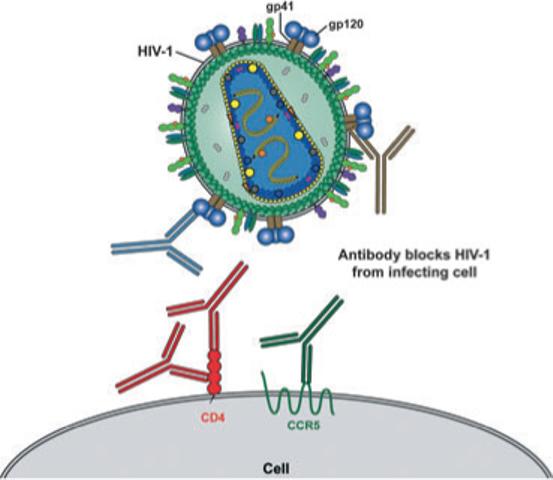
Schematic of HIV-1 and the cellular receptors involved in viral entry. The HIV-1 envelope glycoprotein, gp120 (blue), associated with the transmembrane protein gp41 (brown), are exposed on the outside of the virus particles. HIV-1 binds to the primary receptor, CD4 (red) and, following conformational changes, the gp120–CD4 complexes bind the coreceptor, CCR5 (green), which activates gp41 to mediate fusion of the viral membrane to the target cell membrane, leading to viral entry. The exterior viral envelope glycoproteins, gp120 and gp41 are both targets for neutralizing antibodies as are also, potentially, the cellular receptors CD4 and CCR5. Known neutralizing targets are highlighted with a schematic representation of antibodies that are colour-matched with their target proteins.
Structural features of the HIV-1 envelope glycoproteins, neutralizing determinants and immune evasion
The HIV-1 envelope glycoproteins are present as trimers of gp120/gp41 heterodimers on the surface of infectious virus particles [15–20]. Free gp120 glycoproteins, once shed from the functional spike, can be divided into three antigenic faces: the non-neutralizing, silent and neutralizing faces. The non-neutralizing face of gp120 is recognized by antibodies that bind to gp120 epitopes not exposed in the context of the functional trimer. The neutralizing face binds most known NAbs. The so-called ‘silent face’, which is composed of V4 and V5 variable determinants, is heavily glycosylated and thus is poorly immunogenic. One exceptional broad neutralizing antibody, 2G12, binds to a complex carbohydrate epitope derived from three glycans located in this region [21].
HIV-1 has developed many sophisticated mechanisms to evade Env-directed antibody responses efficiently during natural infection. These include shrouding well-conserved structures by glycan shielding [22] and immunodominant variable loops [23], and masking of vulnerable receptor-binding sites by conformational and steric constraints [24]. In addition, monomeric gp120 is readily shed from virus particles, which stimulates the production of antibodies that bind protein surfaces that are not exposed on the intact Env trimer [25, 26]. Unmodified monomeric gp120 therefore holds little promise as a vaccine component, highlighted by the disappointing, but not unanticipated, results obtained in Vaxgen's human clinical trials [27–29]. As our understanding about Env structure and antigenicity increases, second- and third-generation Env-based immunogens will be designed and tested for their ability to elicit broadly Nabs more efficiently [30].
The HIV-1 envelope glycoproteins are present on the exterior of the virus and due to their critical role for viral entry and their exposed location at the surface, they are the major targets for NAbs [31]. The ability to re-elicit broadly NAbs through vaccination is a major aim of many HIV-1 vaccine development projects; however, the paucity of broadly NAbs highlights the challenge of this goal. To date only four rare broadly NAbs have been identified (Fig. 2), all elicited by chronic HIV-1 infection [32]. On the HIV-1 exterior glycoprotein gp120, the antibody IgGb12 binds to the CD4-binding site (CD4BS) and cross competes with the primary HIV-1 receptor, CD4 [33] and with the weakly neutralizing antibody F105 [34]. A recent study shows that the IgGb12 binds essentially to the outer domain of the HIV-1 gp120 core glycoprotein [35, 69]. The other gp120-directed Nab, 2G12, binds to a cluster of glycans on the gp120 outer domain [21]. The recent crystal structure of Fab fragment of 2G12 in complex with Man9Glc-NAC shows that this antibody adopts a highly unusual domain-exchanged dimeric structure that produces several antibody combining sites in proximity to one another [36]. The gp41-directed antibodies, 2F5 and 4E10, bind to a region close to the viral membrane termed the membrane proximal region (MPR) that is hydrophobic and highly conserved across clades [37, 38]. The recent structure of 2F5 in complex to its epitope reveals that the 2F5 antibody bound to only one face of an extended peptide in a cleft between the heavy and light chain [39]. The structure of 4E10 with its epitope reveals that a helical peptide that bound at the base of the CDRH3 [40]. Another antibody, D5, binds to a region in the gp41 heptad repeat 1 (HR1) and displays limited neutralization breadth and potency, raising issues regarding the accessibility of this epitope on primary isolates [41]. These antibodies and their relevant properties are summarized in Table 1 and are further reviewed in Ref. [42].
Fig. 2.
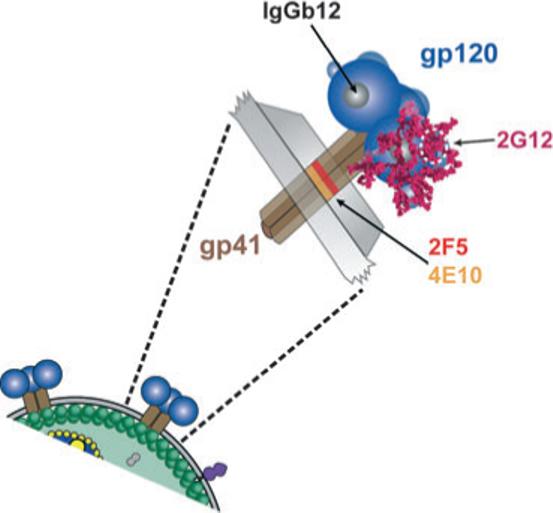
Schematic representation of the functional spike and the binding sites of broadly neutralizing antibodies on the viral envelope glycoproteins. The exterior envelope glyco-protein, gp120 (blue), and the transmembrane glycoprotein, gp41 (brown) are shown as noncovalently associated trimers on the surface of the virus (below) and expanded to reveal more details of the spike (above). The membrane proximal epitopes for the two broadly neutralizing gp41 antibodies; 4E10 (orange) and 2F5 (red) are shown on the trimeric stalk of gp41 external to the viral membrane. The two broadly neutralizing gp120 antibodies are 2G12 and IgGb12. 2G12 recognizes a carbohydrate cluster (encircled in white dashes) on the N-linked glycans (magenta) of gp120, which are shown on one gp120 monomer. The antibody IgGb12 recognizes a discontinuous epitope that overlaps with the recessed CD4-binding site on gp120 (grey area) and is shown on one monomer.
Table 1.
Prototypic HIV-1 Neutralizing antibodies
| Target | Target site | Antibody | Reference | Potency/breadth |
|---|---|---|---|---|
| Virus | ||||
| gp120 | CD4BS | IgGb12 | Burton et al. [33] | Strong/broad |
| F105 | Posner et al. [34] | Weak/narrow | ||
| Glycan | 2G12 | Trkola et al. [21] | Moderate/broad | |
| V3 | 447−52D | Gorny et al. [47] | Strong/narrow | |
| CD4i | 17b | Thali et al. [50] | Weak/narrow | |
| 412D | Raja et al. [51] | Weak/narrow | ||
| X5 | Moulard et al. [52] | Weak/narrow | ||
| gp41 | MPR | 2F5 | Muster et al. [37] | Moderate/broad |
| 4E10 | Zwick et al. [38] | Weak/broad | ||
| Z13 | Zwick et al. [38] | Weak/narrow | ||
| HR1 | D5 | Miller et al. [41] | Weak/narrow | |
| Host protein | ICAM-1 | 15.2 | Rizzuto et al. [81] | Weak/narrow |
| Cell surface | CD4 | 5A8/TNX-355 (hu) | Burkly et al. [93], Kuritzkes et al. [10] | Moderate/broad |
| CCR5 | CCR5mab004 | http://www.hgsi.com | Moderate/broada | |
| 2D7, PA14/Pro 140 (hu) | Olson et al. [11], Lee et al. [12] | Moderate/broadb | ||
| CXCR4 | 12G5 | Strizki et al. [113] | Moderate/broadb | |
| CXCR4 | http://www.nwbio.com | Moderate/broadb |
Effective against CCR5 utilizing HIV-1.
Effective against CXCR4 utilizing HIV-1.
Because the four broadly Nabs target relatively well-conserved regions of HIV-1 Env, it is conceivable that some or all of these antibodies could be used as therapeutic compounds for prevention of mother-to-infant transmission of HIV-1 or as components of microbicide formulations. In a nonhuman primate study modelling neonatal transmission, a cocktail of three NAbs-mediated sterilizing protection of SHIV-challenged infants by mucosal routes [43]. Success in this and other passive immunization studies in nonhuman primates have lead to the initiation of at least one clinical trial designed to inhibit mother-to-child transmission. However, the concept has not gained wide acceptance due to the efficacy, ease and low expense of the reverse transcriptase inhibitor, nevarapine [44]. Recent studies performed in chronically HIV-1-infected humans passively infused with the three broadly neutralizing monoclonals, 2G12, 2F5 and 4E10, demonstrated that transient inhibition and escape of viral replication were observed only to 2G12 [45]. These data were consistent with earlier animal models, which demonstrated that individual monoclonals, at relatively high concentrations, prevent infection if administered before or just after viral challenge [4, 6, 44]. If antibody was administered after the establishment of viral infection, escape from a single antibody was extremely rapid and generally clonal or oligoclonal. This is presumably due to the high mutation rate of HIV/SIV that generates many escape mutants, some of which are fit for replication. As a result, the most fit virus predominates after several rounds of replication following escape. Besides the more broadly neutralizing determinants described above, other regions responsible for neutralization activity in a more restricted manner are the immunodominant variable loop 3 (V3) and, to a lesser extent, the V2 loop. The V3 loop of gp120 elicits antibodies that are often more strain-restricted in terms of neutralization, although some antibodies directed against conserved determinants of V3 bind monomeric gp120 from multiple strains, such as 447−52D [47, 48]. The recent crystal structure of the HIV-1 gp120 core containing the entire V3 loop reveals that, once triggered by binding with CD4, the protruding V3 loop is highly accessible on the gp120 monomer, perhaps explaining the immunodominance of this region [49]. Another set of antibodies, such as 17b [50], 412D [51] and X5 [52] are referred to as CD4-induced (CD4i) antibodies and recognize the highly conserved coreceptor-binding site on the gp120 core (Table 1). However, occlusion of most CD4i epitopes due to steric hindrance limits the neutralization activity displayed by the CD4i site antibodies [52, 53]. This is consistent with the recent observation that such antibodies are at relatively high levels in patient sera [54], and it is likely that in vivo selection pressure is exerted to select for viruses that occlude the conserved gp120 region involved in binding to the monomorphic coreceptor, CCR5.
HIV-1 envelope glycoprotein immunogen design
Most successful antiviral vaccines consist of either live-attenuated or inactivated viral particles. However, live attenuation of the HIV-related simian immunodeficiency virus (SIV), resulting in protective responses without resulting pathogenicity has not been accomplished [55], raising safety concerns that make human trials intractable. Similarly, chemical inactivation of HIV-1 has not yet generated broadly protective antibody responses. Therefore, investigators turned to subunit, Env-based immunogens a means of eliciting broadly NAbs. The use of monomeric gp120 or peptides derived from the immunodominant V3 loop of gp120 generates type-specific, but not broadly NAbs, likely due to occlusion of functionally conserved V3 epitopes on most circulating isolates. The failure of gp120 to elicit efficiently broadly NAbs has shifted design efforts to engineering soluble versions of the Env spike to recapitulate properties of the functional trimer. We will briefly summarize such efforts here, but for more extensive treatment we refer readers to other more comprehensive reviews focused on HIV-1 Env-based immunogen design [18, 30, 56].
Soluble versions of the spike, containing full-length gp120 covalently linked to selected versions of the gp41 domain, have been generated and are commonly referred to as ‘gp140 molecules’. To name a few iterations on this common theme: C-terminal heterologous trimerization motifs were added to gp120-gp41 molecules [57, 58], cysteine-pair linkages were added to covalently linked gp120 to gp41 [59], flexible inter gp120-gp41 linkers were generated as another means to covalently link gp120 to gp41 [60], variable loop deletion of gp140 molecules to expose cryptic epitomes were performed [61], deletion of the fusion peptide and gp41 immunodominant cysteine loop to remove nondesired epitopes were created [62], solid-phase proteoliposomes containing full-length Env embedded in a reconstituted lipid bilayer were made [63] and consensus sequence-derived gp140 soluble spike mimetics were characterized and tested as immunogens [reviewed in Ref. 30]. Amongst other approaches to mimic properties of the functional spike, chemically inactivated virions and virus-like particles [reviewed in Ref. 30, 64] as well as CD4–gp120 complexes designed to reveal cryptic, transition state epitopes displayed by Env during the entry process can be included [65]. Although slight relative improvements in neutralization breadth and potency were reported in some of these studies, none of the designs by themselves appear to generate sufficient neutralization breadth to impact on reducing HIV-1 diversity worldwide in a vaccine setting.
To better elicit broadly Nabs using Env-based monomeric gp120 subunits, several groups have devised rational and innovative strategies to improve on the natural properties of Env. Hyperglycosylated derivatives of gp120/gp140 were designed that attempt to focus the immune response to conserved epitopes that form part of the CD4BS [66, 67] and render immuno-silent variable and non-neutralizing elements of gp120. Structure-based attempts to stabilize gp120 in the CD4-bound conformation by site-direct mutagenesis and thermodynamic analysis have been attempted, but to date limited improvements in the elicitation of NAbs has been reported [65]. The availability of structural information presents unique opportunities to target discrete elements of Env for immunogen design. The most recent example of this are broadly neutralizing determinants within the gp41 MPR that has been crystallized by two different groups and consists of the MPR-directed, broadly NAbs 2F5 and 4E10 in complex with their cog-nate linear epitopes. Based upon the gp41 peptide conformations defined in the structures, constrained peptides and other novel means to stabilize the MPR sequences and present them in the lipid context proximal to this region are being designed and evaluated as vaccine candidates. For example, the MPR-based immunogens are being arrayed on carrier molecules (HepB surface antigen) to increase B-cell responses as well as to provide T-help and a heterologous lipid membrane. A parallel approach is structure-assisted scaffolding of the 2F5 and 4E10 epitopes on heterologous proteins containing similar folds as those adopted by the extended loop structure of the 2F5 epitope or the helical conformation of the 4E10 epitope [39, 68].
For gp120-based approaches alternative to stabilizing the CD4-bound conformation described above, the structure of the broadly neutralizing CD4BS antibody, IgGb12, in complex with core gp120 was recently reported. The structural definition of this conserved gp120 surface should aid greatly in designing immunogens intended to re-elicit this type of broadly neutralizing antibody [69]. The structure of the intact trimeric, functional spike might be the most critical structure to obtain for structure-based envelope glyco-protein immunogen design, but to date no such structure has been forthcoming. It should be said that although rational, structure-based immunogen design is an inviting new path, if is far from proven that it will lead to an effective HIV-1 vaccine.
Other factors to consider in protein subunit-based vaccine development is modulation of the immune system by selected adjuvants. Improved oil-in-water adjuvants with and without immune-activating compounds such as the Toll-like receptor 4 (TLR-4) agonist MPL are under development and evaluation by GlaxoSmithKline or their collaborators [70]. Other TLR agonists and other molecular adjuvants that target innate pathways to activate the adaptive immune response are exciting new areas of vaccine development that might be capable of eliciting both antibody and cellular responses from protein immunogens. Such approaches may impact favourably on HIV-1 vaccine design in the future, especially if the adjuvant conjugated directly to the protein antigen [71–73].
The viral receptors as antibody targets to inhibit HIV entry
One of the most daunting hurdles to overcome in developing an effective HIV-1 vaccine is the viral heterogeneity, which increases with every passing day. Viral heterogeneity is a concern for both the breadth of cellular immune responses, which also depends upon polymorphic MHC restrictions, and for the coverage required by broadly NAbs. Because the virus is extremely responsive to selection pressures, unless the vaccine exerts pressure akin to the level displayed by the triple combination antiretroviral drugs, a transient drop in viral transmission may be achieved, but may only mark the evolution of a new, vaccine-resistant viral branch point. Initially, such a branch might be more limited in terms of viral diversity and perhaps then easier to control by a second-generation vaccine if the correlates and mechanisms of protection from the first generation vaccine are well understood.
A vaccine eliciting ‘neutralizing antibodies’ that are directed at monomorphic host molecules would eliminate many concerns of viral heterogeneity. Such antibodies, with the potential capacity to inhibit entry of diverse HIV-1 strains using the same invariant receptor, would be less affected by HIV heterogeneity. If efficiently elicited, such antibodies would not be directly ‘neutralizing’ virus in the classical virological jargon, but would inhibit viral entry. For ease of discussion, all HIV entry-inhibiting antibodies will be referred to as Nabs in this review. Vaccines elicited against self-proteins will encounter many obstacles of basic immunology and safety concerns, but if they display promise in small animals, selected approaches could be assessed for safety and efficacy in nonhuman primates. If proof-of-principle can be demonstrated in nonhuman primates, then such approaches could be considered for testing in infected humans, who harbour HIV escape variants from all other forms of therapy. Importantly, such approaches do (potentially) take most aspects of viral diversity out of the picture if the cellular target is properly selected. At this juncture, there are primarily two relevant cellular targets directly involved in the HIV-1 entry process: the primary receptor, CD4 and the viral coreceptors, chemokine receptors CCR5 or CXCR4. As described below, each of these cellular proteins has received some attention as therapeutic targets and, to a limited extent, CCR5 has been examined in a small number of preclinical studies as a vaccine target. Other potential targets are lectin-like molecules found on DCs, such as the C-type lectin DC-SIGN [74]. Because the precise role of DC-SIGN in mediating HIV-1-specific attachment to DCs and transport in vivo is currently undergoing revision [75] we will not discuss this area in detail here. Suffice it to say that it is likely that lectins may mediate DC interaction with glycosylated viruses such as HIV-1 and that antibodies against such cellular molecules could have therapeutic value in interfering with HIV-1 infection at these key portals of entry in the submucosa. The ability of cyanovirin, a lectin-like protein isolated from natural sources, to inhibit HIV-1 entry in vitro provides proof-of-principle for this concept [76, 77]. Selected antibodies against relevant cellular molecules are summarized in Table 1.
Because several strategies need to be explored in the quest to develop effective means to inhibit HIV-1 replication either via a vaccine or, also relevant to this review, via therapeutic antibodies, cellular molecules that are incorporated into the virus particle are also worth limited discussion regarding their relevance as potential neutralization determinants. Early studies using SIV propagated in human cells suggested that xeno-immunization provided some degree of protection against SIV [78, 79]. Subsequent studies suggested that this response was in part mediated by antibodies against MHC class I and II molecules incorporated into the membrane of the virus. Other molecules, including adhesion molecules such as the intracellular adhesion molecule 1, ICAM-1, and its primary receptor leucocyte function-associated molecule 1, LFA-1, have also been shown to be incorporated into the HIV-1 membrane and to modulate the neutralization sensitivity of HIV-1 [80, 81]. The potential to target molecules that are incorporated into HIV-1 particles represent at least a theoretical alternative approach for interfering with HIV-1 entry (see below).
CD4 as a soluble immunotherapeutic or as a therapeutic antibody target
The primary virus receptor, CD4, is a member of the immunoglobulin super family and is a major phenotypic marker and functional protein on T-helper cells. CD4 consists of four extracellular domains (D1–D4) and the CDR2 loop of the first domain (D1) plays a critical role in interaction with the HIV-1 envelope glycoprotein gp120 during viral entry [82]. In the early 1990s, a soluble form of CD4 (sCD4) was under investigation as a therapeutic drug. Initial attempts at using sCD4 as therapeutic inhibitor of HIV-1 infection were met with limited success likely due the discovery that primary isolates were much more resistant to the neutralizing capacity of sCD4 than had been previously appreciated [83, 84]. Alternative versions of sCD4 have been made to improve its neutralizing activity by grafting the D1 and D2 domains onto selected IgG constructs to create immunoglobulin fusions containing two or four CD4BS (dimeric and tetrameric CD4). The most promising CD4-based fusion protein, PRO542 [85], is a tetravalent CD4–Ig construct that contains the D1 and D2 domains of human CD4 genetically fused to heavy and light chain constant regions of human IgG2κ. This molecule has been shown to potently inhibit HIV-1; besides increased valency and avidity, the greater conformational flexibility in PRO542 may contribute to its enhanced antiviral activity [85] via activation of complement or opsonization in vivo. In preclinical studies, PRO542 is well tolerated at a dose of 25 mg kg−1 and reduces the viral load significantly in advanced disease HIV-1 patients [87–89].
In an alternative but related strategy, antibodies against CD4 were characterized and shown to exhibit potent inhibitory activity of HIV-1 entry by both directly blocking gp120–CD4 interaction or interfering with fusion [90, 91]. One of these antibodies, the calcium-dependent anti-CD4 antibody Q425, recognizes an epitope in the CD4 domain 3 and was recently crystallized in complex with CD4 [92]. Tanox has developed a humanized version of a murine IgG4 monoclonal antibody, TNX-355, which binds to the D2 domain of CD4 [10, 93]. The antibody, 5A8, was originally isolated from a mouse immunized with human sCD4. As the TNX-355 epitope lies outside the D1-located binding site of class II molecules on CD4, it apparently does not interfere with the normal immunological functions of CD4. A single dose of TNX-355 reduced viral load and increased CD4+ T-cell counts in HIV-1-infected patients and phase II clinical trial results showed a more than 2 log reduction in viral load after 24 weeks of treatment [10]. TNX-355 may have an adjunct role in salvage therapy in the near future, if the clinical data continues to be positive.
CCR5 as a therapeutic or vaccine target
Another cellular molecule that has been explored as an antibody target is the chemokine receptor 5, CCR5, which is the most important HIV-1 coreceptor in vivo. Host genetics studies on HIV-1 susceptibility have identified interesting CCR5 polymorphisms in the human population that reduce the risk of HIV infection [94, 95]. Furthermore, seronegative individuals have an increased frequency of an N-terminal 32 residue deletion (CCR5-Δ32) that produces a functionally defective CCR5 molecule [96, 97]. Perhaps because there are several receptors with similar chemokine receptor activity, the CCR5 molecule does not appear essential for immune function and survival, at least in the developed world. Attempts to induce antibodies that block the regions of CCR5 used by HIV-1 to gain entry into the cell are described in more detail below, but, as a prelude, we will summarize the rationale to target CCR5 with low-molecular weight compounds.
Chemokine receptor 5 and to a lesser extent, the other HIV-1 coreceptor, CXCR4, have been under intense targeting by several pharmaceutical companies for small molecular weight inhibitors. CCR5 is one member of a large family of G-protein-coupled, 7-membrane-spanning receptors (GPCRs). CCR5 binds and signals via the β-chemokines MIP-1α, MIP-1β and RANTES [98, 99] and has become a preferred target for GPCR drug development due to the existence of a small number of CCR5-Δ32 homozygotes that express no functional CCR5 molecules on their cells, yet have no observable pathological phenotype [94, 97]. Supporting the null phenotype for loss of CCR5 in the human condition, CCR5 knock out mice demonstrated no severe developmental nor immunological defects [100]. A recent set of studies suggest that the presence of anti-CCR5 antibodies in a subset of HIV-infected long-term nonprogressors induce long-lasting downregulation of CCR5, perhaps contributing to their nonprogressor clinical phenotype [101–103].
There has been growing interest over the therapeutic potential of CCR5-targeted compounds as new anti-HIV-1 agents [104]. Of the small molecular weight CCR5 inhibitors, Schering's SH-C and SH-D compounds are the furthest along in Phase 1 clinical trials and Pfizer's also has a CCR5-targeted drug in clinical development [9]. The conserved helical trans-membrane structure in the membrane spanning region of many GPCRs is where most CCR5 inhibitory compounds appear to bind [105] and may contribute to toxicity concerns that caused Merck to abandon its CCR5 drug development programme. Furthermore, gp120–CD4 complexes likely do not interact with the hydrophobic helical pocket that is the target for the most noncompetitive inhibitory drugs. Therefore, not surprisingly, HIV-1 can escape from inhibition mediated by many of the compounds [106–108]. In this regard, an antibody that binds in a competitive manner, with a much larger protein–protein footprint, might have improved efficacy compared with the low-molecular weight drugs.
Exploring the antibody approach, Human Genome Sciences recently identified a potent anti-CCR5 antibody, CCR5 mab004, which inhibits entry from representative HIV-1 isolates from clades A–G, without inducing either cell signalling or apparent cell toxicity [13]. This antibody is now in Phase I clinical trial and efficacy data should be forthcoming in the near future. Progenics is also exploiting the exquisite specificity and large contact surface of antibody to develop an anti-CCR5 humanized antibody, Pro140 (originally called PA14), which is currently under evaluation in Phase I clinical trial. The PA14 antibody, similar to 2D7 antibody, was raised initially in mice against cells expressing CCR5. These antibodies bind to a complex determinant composed of the human CCR5 extracellular loops (ECLs), predominantly the second ECL and effectively block HIV-1 infection of target cells [11, 12]. In general, the anti-CCR5 antibodies against ECL loops are potent inhibitors of HIV-1 entry and might function by inhibiting direct binding of CD4-activated gp120 or inhibit postbinding conformational changes or oligomerization of CCR5 [109]. Antibodies against the CCR5 N-terminus also block entry with varying degrees of activity, but generally are less potent than those against the ECLs [11, 110, 111]. Once the in vivo efficacy of the CCR5 antibodies in clinical trials is determined, they may be considered as components of a microbicide.
Only one potent anti-CXCR4 antibody (12G5) that inhibits HIV-1 entry has been well characterized. The 12G5 murine monoclonal recognizes an epitope in ECL2 of CXCR4 [112, 113]. However, in the past several years, with their success against cancer targets, humanized or fully human monoclonal antibodies have regained favour as therapeutic drugs due to their exquisite specificity, relative ease of production and long in vivo half-life (e.g. Herceptin against the Her2 receptor for breast cancer therapy (114]). In that light, Northwest Pharmaceuticals is developing an anti-CXCR4 humanized monoclonal antibody for multiple therapeutic applications (Table 1).
Because CCR5 is considered dispensable for human survival, in principle, active immunization against CCR5 could induce a long-lasting immunity against HIV-1 infection, either in a therapeutic modality or potentially as a prophylactic vaccine. Several studies have attempted to generate anti-CCR5 inhibitory responses, including DNA priming and boosting with CCR5-derived peptides [115], or bacterially expressed fragments of CCR5 displayed on particles [116] with limited success. Another study used the 2D7 antibody to isolate a peptide by phage display panning. The peptide displayed homology for the CCR5 ECL2 and it was reported that the peptide could elicit antibodies that then bound CCR5 and inhibited HIV-1 infection in vitro [117].
Recent advancement to generate conformationally intact CCR5 either as proteoliposomes [118] or as soluble molecules in lipid: detergent mixtures [119, 120] will allow immunization of small animals with fully native CCR5. The goal will be to determine if this approach will better elicit HIV inhibitory antibodies than have been currently generated by other means [121–123]. However, the difficulties of active immunization against a ‘self’ cellular target are obviously of great concern, not underestimating the requirements to keep a 7-membrane-spanning protein in its native conformation throughout the vaccination process. The initial immunological hurdle to overcome will be to break tolerance to a self-antigen in an adult animal or human. Initially there are several ways to determine if this is feasible in small animal models. For example, immunization with the intact protein, or a fragment of the protein, along with an heterologous T-helper epitope, which in some cases is sufficient to break tolerance [124]. Cross species immunization of CCR5 contained in proteoliposomes (i.e. human CCR5 into mice or rabbits) are alternative strategies to break tolerance. If tolerance to CCR5 can be overcome, then it will be critical to induce anti-CCR5 inhibitory antibodies and not cytolytic cellular responses, which could induce pathology. If anti-CCR5 HIV-1-inhibiting antibodies are elicited in small animals, it remains to be determined if they can be induced in monkeys and mediate protection either as a therapeutic or prophylactic vaccine for SIV. If protection is accomplished in the absence of pathology, then such CCR5 immunogenicity–challenge experiments would be on the critical path to testing in humans.
Other cellular proteins as antibody targets
Historically, examination of HIV or SIV particles has revealed that a myriad of cellular proteins are incorporated into viral particles [125]. A subset of these molecules was shown to mediate protection against SIV challenge in nonhuman primates as discussed immediately below. Antibodies to another abundant molecule incorporated into virions (ICAM-1) can mediate viral neutralization in vitro [81]. Although it is less likely that either of these cellular molecules are practical vaccine or therapeutic targets, for a complete discussion of the ‘HIV-1 neutralizing determinants’ topic, selected examples are worth highlighting. Although outside the scope of this review, microbicide formulations will likely incorporate combination therapy against multiple targets involved in the HIV-1 entry process to achieve broad and potent efficacy [126, 127]. In this light, it is prudent to be aware of all effective inhibitors of HIV-1 entry, including those directed against cellular components.
Xeno- or allo-immunization against HLA class I and II molecules
In addition to cellular proteins that serve as viral receptors essential for HIV-1 entry into host cells, there are a number of other cellular molecules incorporated in the viral membrane, which may enhance HIV-1 infectivity or modulate pathogenesis (Fig. 3). The majority of host cell-derived membrane proteins are excluded when retroviruses bud from the plasma membrane; however, early studies on human T-cell leukaemia virus type I (HTLV-1), avian leukosis virus and HIV-1 demonstrated that some cellular molecules are selectively incorporated into the viral membrane [128–130]. The potential benefit of targeting host-derived molecules through vaccination became apparent when it was shown that fixed SIV-infected C8166 cells provided protection against SIV challenge [131] and that a similar level of protection could be achieved by using fixed, but uninfected, cells alone [132]. This strongly suggested that protection against SIV in this model was mediated by immune responses directed against cellular proteins rather than against viral determinants. Soon thereafter, Arthur et al. showed that β2-microglobulin (β2m) as well as the α- and β-chains of human lymphocyte antigen (HLA) molecules were present in HIV-1, HIV-2 and SIV virus preparations grown in H9 cells (a human T-cell line) [133]. This has later been confirmed by several investigators [128, 134–136] and attempts to understand the role of these processes in the pathogenesis of HIV-1 are still ongoing. Reactivity to cellular proteins incorporated in the virus particles appear to explain early results that monkeys immunized with purified inactivated SIV preparations were protected against subsequent challenge with SIV [78]. Although a contribution from cellular immune responses cannot be ruled out, it is likely that elicitation of HLA-directed antibody responses explains most of the protection. So far, the relative roles of MHC class I and class II molecules as protective antibody targets remains unclear. Analysis of sera from protected monkeys immunized with fixed uninfected cells demonstrated that there was positive correlation of protection with antibody response to the HLA class I molecule [137]. However, in another study, cynomolgus macaques were immunized with purified β2m, HLA class I or class II proteins prior to SIV challenge and all animals immunized with the class II immunogens were protected, whilst animals immunized with β2m or HLA class I immunogens were not protected despite high antibody titres against these antigens [138]. Thus, it remains possible that both HLA class I and class II molecules induce xeno-antibodies that can mediate some level of protection against SIV.
Fig. 3.
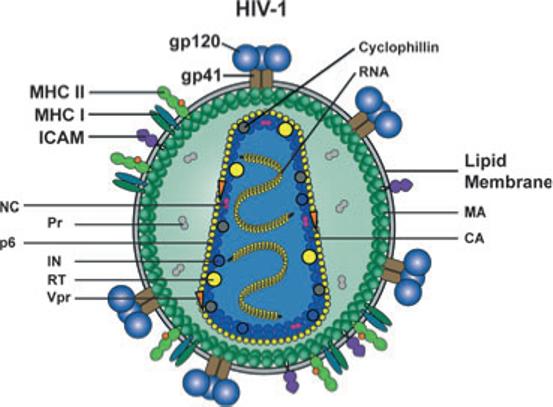
Schematic representation of the HIV-1 particle and its protein and genomic RNA contents. Two copies of the viral positive strand RNA genome are packaged into the particle. Viral enzymes; reverse transcriptase (RT), integrase (IN), protease (Pr) and structural proteins; capsid (CA), nucleocapsid (NC), matrix (MA) and p6 are inside the particle together with the viral regulatory protein Vpr and the cellular protein cyclophilin. The virus is enveloped by a host cell-derived lipid membrane into which trimers of the viral transmembrane glycoprotein gp41 is inserted. Three monomers of the exterior envelope glycoprotein gp120 are noncovalently associated with gp41. Several cellular proteins are also incorporated into the viral membrane with relative abundance, including MHC class I and II molecules and intracellular adhesion molecule-1 shown here.
Results describing a role for allo-antibodies in HIV-1 protection are more sparse [139], but this approach is also being considered as a vaccine strategy. Allo-antibody responses against HLA-B62 and HLA-DR4 were detected in individuals receiving fixed inactivated HIV-1 particles as immunotherapy (consistent with the HLA types of the producer cell line), whilst no such responses were detected in HLA-B62- and HLA-DR4-positive individuals suggesting that tolerance had not been broken [140]. As discussed by others [141], the rationale for using HLA molecules as components of an HIV-1 vaccine is that transmitted HIV-1 particles would carry the donor's HLA molecules and by establishing an allo-response through prophylactic vaccination against an array of nonself HLA molecules, the rate of transmission could possibly be curbed. The observation that intrapartum transmission of HIV-1 correlated with the degree of MHC class I concordance between mothers and infants in a study in Nairobi [142] lends support to this hypothesis. It is known that the allogeneic response is one of the strongest antigen-specific immune responses and that this response is already well developed in the newborn. Furthermore, immune responses to allo-antigens could potentially eliminate both virus-infected cells and free particles, which may be important as it is not known whether HIV-1 transmission is mediated by cell-free virus or by infected cells. Both viral sources are present in vaginal fluid and semen [143]. However, an obvious limitation to the allo-immunization approach is that, in contrast to other cellular molecules, there is a high degree of HLA polymorphism in the human population. It would therefore be necessary to immunize against multiple HLA haplotypes to obtain broad protection against diverse HIV-1 infected individuals.
Strategies to immunize against MHC molecules include the use of fixed cells [131], plasmid DNA [144] or inactivated virus particles [78, 79, 140, 145]. Generally, purified protein administered in adjuvant provides the most potent humoral immunity. Protocols for expressing, purifying and refolding HLA molecules have advanced over the past years, but many challenges remain. Soluble HLA class I molecules are relatively easy to refold and therefore might be more amenable to presentation as an immunogen [146, 147]. In contrast, only a subset of HLA class II alleles have been successfully re-folded [148–150]. Current efforts to devise protocols that allow native folding of HLA molecules and use of such proteins in preclinical immunization experiments will show if this approach is promising for the development of an HIV-1 vaccine.
Targeting cell adhesion molecules incorporated into HIV-1
In addition to MHC class I and II molecules, several other cellular proteins are incorporated into HIV-1 particles [125, 134, 135]. The repertoire and amount of cellular proteins incorporated into HIV-1 depend on the source of cells from which the virus is isolated from and the activation state of these cells. During natural infection, most free virus is likely to be produced from activated T cells [151, 152], in which many cell surface molecules are upregulated. Some of these proteins are cell adhesion molecules, including ICAM-1 (CD54) and LFA-1 (CD11a), whose presence on viral particles modulates the virus’ neutralization sensitivity [80, 81]. Other molecules, such as CD86, are also preferentially incorporated into virus particles and may influence HIV-1 pathogenesis if these molecules are biologically active [134, 153]. The presence of ICAM-1 on HIV-1 particles was shown to endow the virus with an increased ability to attach to target cells and to initiate infection [134, 154]. Despite increased infectivity by ICAM-1 containing HIV-1, viral entry could be efficiently inhibited by antibodies against ICAM-1 [80] raising the possibility that ICAM-1 could be considered a target for passive therapeutic intervention or, less likely, as prophylactic vaccine target. The large ‘sink’ of ICAM-1 in vivo, the critical function of ICAM-1 in many biological process and potential pathogenicity, however, make both of these prospects unlikely. Perhaps inclusion of anti-ICAM-1 antibodies in a topical microbicide could be considered. Targeting other nonpolymorphic cellular molecules incorporated in HIV-1 particle is associated with similar concerns, but could be revisited if other more promising approaches do not demonstrate therapeutic or prophylactic efficacy.
Concluding remarks
In this review, we have described viral and cellular molecules that potentially could be targets for antibodies, or antibody-related molecules, to block HIV-1 entry into susceptible target cells and decrease the spread of HIV-1 in the human population. As described, a prophylactic vaccine against conserved but exposed epitopes on the HIV-1 envelope glycoproteins gp120 or gp41 is the best option to limit newly acquired infections. Innovative approaches to generate broadly neutralizing activity via subunit Env-based vaccine strategies represent the major focus of current research and development in this area. However, the difficulties in generating antibodies of sufficient neutralizing breadth and potency against these viral targets should be obvious from the selected approaches and studies described in this review. With the exception of other chronic RNA viruses such as Hepatitis C virus, for which a prophylactic vaccine does not yet exist either, other viruses do not generally pose such daunting requirements on vaccine development as does HIV-1. The successful influenza virus vaccine, for example, mediates essentially subtypespecific protection that requires a close match between the vaccine and circulating strain. This vaccine, although effective, does not elicit broadly protective antibodies or overcome the hurdle of viral variability and hence has to be evaluated each year for homology to the predicted circulating viral strain. The need to develop preventative strategies against the wide array of HIV-1 isolates seeded in the human population is more urgent now than ever since the epidemic continues to spread in many regions of the developing and developed world. For individuals already infected by HIV-1, drugs exist, but they are not available in much of the developing world. Drug-resistant escape mutants continue to emerge in patients undergoing HAART and those of sufficient fitness can transmit viral infection. Therefore, alternative possibilities of interfering with HIV-1 entry in a therapeutic modality using antibodies that target cellular molecules involved in the HIV-1 entry process or cellular molecules that are associated with virus particles may merit consideration as future salvage therapies as discussed in this review.
Acknowledgements
We thank Brenda Hartman and Michael Cichanowski at the VRC/NIH for expert assistance with figures. RTW and SP are supported by the NIAID intramural research program and GKH is supported by the International AIDS Vaccine Initiative (IAVI), the Swedish International Development Agency (SIDA)/Department of Research Cooperation (SAREC) and the Swedish Research Council.
Footnotes
Conflict of interest statement
No conflict of interest was declared.
References
- 1.Berman PW, Gregory TJ, Riddle L, et al. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990;345:622–5. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- 2.Ferrantelli F, Rasmussen RA, Hofmann-Lehmann R, Xu W, McClure HM, Ruprecht RM. Do not underestimate the power of antibodies – lessons from adoptive transfer of antibodies against HIV. Vaccine. 2002;20(Suppl 4):A61–5. doi: 10.1016/s0264-410x(02)00389-4. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann-Lehmann R, Vlasak J, Rasmussen RA, et al. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J Virol. 2001;75:7470–80. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR, Lewis MG, Stiegler G, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–18. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascola JR, Stiegler G, VanCott TC, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 6.Veazey RS, Shattock RJ, Pope M, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarin A, Clotet B, Cooper D, et al. Efficacy of enfuvirtide in patients infected with drug-resistant HIV-1 in Europe and Australia. N Engl J Med. 2003;348:2186–95. doi: 10.1056/NEJMoa035211. [DOI] [PubMed] [Google Scholar]
- 8.Hazuda DJ, Young SD, Guare JP, et al. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–32. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- 9.Westby M, van der Ryst E. CCR5 antagonists: host-targeted antivirals for the treatment of HIV infection. Antivir Chem Chemother. 2005;16:339–54. doi: 10.1177/095632020501600601. [DOI] [PubMed] [Google Scholar]
- 10.Kuritzkes DR, Jacobson J, Powderly WG, et al. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis. 2004;189:286–91. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- 11.Olson WC, Rabut GE, Nagashima KA, et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol. 1999;73:4145–55. doi: 10.1128/jvi.73.5.4145-4155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee BM, Sharron C, Blanpain BJ, et al. Epitope mapping of CCRS reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–26. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 13.Roschke V, Moore PA. Characterization of a Panel of Novel Human Monoclonal Antibodies that Specifically Antagonize CCR5 and Block HIV-1 Entry.. 44th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC).2004. p. #2871. [Google Scholar]
- 14.Chan DC, Kim PS. HIV entry and its inhibition. Cell. 1998;93:681–4. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 15.Center RJ, Lebowitz J, Leapman RD, Moss B. Promoting trimerization of soluble human immunodeficiency virus type 1 (HIV-1) Env through the use of HIV-1/simian immunodeficiency virus chimeras. J Virol. 2004;78:2265–76. doi: 10.1128/JVI.78.5.2265-2276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Center RJ, Schuck P, Leapman RD, et al. Oligomeric structure of virion-associated and soluble forms of the simian immunodeficiency virus envelope protein in the prefusion activated conformation. Proc Natl Acad Sci U S A. 2001;98:14877–82. doi: 10.1073/pnas.261573898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chertova E, Bess JW, Jr, Crise BJ, et al. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU). Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2002;76:5315–25. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science. 1998;280:1884–8. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 19.Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog. 2006;2:e83. doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu P, Liu J, Bess J, Jr, et al. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature. 2006;441:847–52. doi: 10.1038/nature04817. [DOI] [PubMed] [Google Scholar]
- 21.Trkola A, Purtscher M, Muster T, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–8. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–12. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 23.Goudsmit J, Debouck C, Meloen RH, et al. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988;85:4478–82. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwong PD, Doyle ML, Casper DJ, et al. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature. 2002;420:678–82. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 25.Moore JP, Sodroski J. Antibody cross-competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–72. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyatt R, Desjardin E, Olshevsky U, et al. Analysis of the interaction of the human immunodeficiency virus type 1 gp120 envelope glycoprotein with the gp41 transmembrane glycoprotein. J Virol. 1997;71:9722–31. doi: 10.1128/jvi.71.12.9722-9731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burton DR, Desrosiers RC, Doms RW, et al. Public health. A sound rationale needed for phase III HIV-1 vaccine trials. Science. 2004;303:316. doi: 10.1126/science.1094620. [DOI] [PubMed] [Google Scholar]
- 28.Graham BS, Mascola JR. Lessons from failure – preparing for future HIV-1 vaccine efficacy trials. J Infect Dis. 2005;191:647–9. doi: 10.1086/428406. [DOI] [PubMed] [Google Scholar]
- 29.Ltd A. I. HIV gp120 vaccine – VaxGen; AIDSVAX, AIDS-VAX B/B, AIDSVAX B/E, HIV gp120 vaccine – Genentech; HIV gp120 vaccine AIDSVAX – VaxGen; HIV vaccine AIDS-VAX – VaxGen. Drugs R D. 2003;4:249–53. doi: 10.2165/00126839-200304040-00007. [DOI] [PubMed] [Google Scholar]
- 30.Phogat S, Wyatt R. Rational modification of HIV-1 envelope glycoproteins for immunogen design. Curr Pharm Des. 2007;13:213–27. doi: 10.2174/138161207779313632. [DOI] [PubMed] [Google Scholar]
- 31.Wyatt R, Kwong PD, Desjardins E, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–11. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 32.Burton DR, Desrosiers RC, Doms RW, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5:233–6. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 33.Burton DR, Pyati J, Koduri R, et al. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–7. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 34.Posner MR, Cavacini LA, Emes CL, Power J, Byrn R. Neutralization of HIV-1 by F105, a human monoclonal antibody to the CD4 binding site of gp120. J Acquir Immune Defic Syndr. 1993;6:7–14. [PubMed] [Google Scholar]
- 35.Kwong PD, Zhou T, Xu L, et al. Functional constraints and antibody recognition of the CD4-binding site on HIV-1 gp120. Antiviral Ther. 2006;11:159. [Google Scholar]
- 36.Calarese DA, Scanlan CN, Zwick MB, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–71. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 37.Muster T, Steindl F, Purtscher M, et al. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–7. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwick MB, Labrijn AF, Wang M, et al. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol. 2001;75:10892–905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ofek G, Tang M, Sambor A, et al. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J Virol. 2004;78:10724–37. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardoso RM, Zwick MB, Stanfield RL, et al. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–73. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Miller MD, Geleziunas R, Bianchi E, et al. A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc Natl Acad Sci U S A. 2005;102:14759–64. doi: 10.1073/pnas.0506927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabel GJ. Immunology. Close to the edge: neutralizing the HIV-1 envelope. Science. 2005;308:1878–9. doi: 10.1126/science.1114854. [DOI] [PubMed] [Google Scholar]
- 43.Baba TW, Liska V, Hofmann-Lehmann R, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 44.Oleske JM. This is no time to stop use of nevarapine to prevent mother-to-child transmission of HIV. AIDS. 2006;20:1059–60. doi: 10.1097/01.aids.0000222079.75867.3e. [DOI] [PubMed] [Google Scholar]
- 45.Trkola A, Kuster H, Rusert P, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005;11:615–22. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura Y, Igarashi T, Haigwood NL, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc Natl Acad Sci U S A. 2003;100:15131–6. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorny MK, Williams C, Volsky B, et al. Human monoclonal antibodies specific for conformation-sensitive epitopes of V3 neutralize human immunodeficiency virus type 1 primary isolates from various clades. J Virol. 2002;76:9035–45. doi: 10.1128/JVI.76.18.9035-9045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartley O, Klasse PJ, Sattentau QJ, Moore JP. V3: HIV's switch-hitter. AIDS Res Hum Retroviruses. 2005;21:171–89. doi: 10.1089/aid.2005.21.171. [DOI] [PubMed] [Google Scholar]
- 49.Huang CC, Tang M, Zhang MY, et al. Structure of a V3-containing HIV-1 gp120 core. Science. 2005;310:1025–8. doi: 10.1126/science.1118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thali M, Moore JP, Furman C, et al. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–88. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raja A, Venturi M, Kwong P, Sodroski J. CD4 binding site antibodies inhibit human immunodeficiency virus gp120 envelope glycoprotein interaction with CCR5. J Virol. 2003;77:713–8. doi: 10.1128/JVI.77.1.713-718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moulard M, Phogat SK, Shu Y, et al. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc Natl Acad Sci U S A. 2002;99:6913–8. doi: 10.1073/pnas.102562599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Labrijn AF, Poignard P, Raja A, et al. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol. 2003;77:10557–65. doi: 10.1128/JVI.77.19.10557-10565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decker JM, Bibollet-Ruche F, Wei X, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med. 2005;201:1407–19. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koff WC, Johnson PR, Watkins DI, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 56.Burton DR, Stanfield RL, Wilson IA. Antibody vs. HIV in a clash of evolutionary titans. Proc Natl Acad Sci U S A. 2005;102:14943–8. doi: 10.1073/pnas.0505126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X, Farzan M, Wyatt R, Sodroski J. Characterization of stable, soluble trimers containing complete ectodomains of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 2000;74:5716–25. doi: 10.1128/jvi.74.12.5716-5725.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X, Lee J, Mahony EM, Kwong PD, Wyatt R, Sodroski J. Highly stable trimers formed by human immunodeficiency virus type 1 envelope glycoproteins fused with the trimeric motif of T4 bacteriophage fibritin. J Virol. 2002;76:4634–42. doi: 10.1128/JVI.76.9.4634-4642.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Binley JM, Sanders RW, Clas B, et al. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J Virol. 2000;74:627–43. doi: 10.1128/jvi.74.2.627-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow YH, Wei OL, Phogat S, et al. Conserved structures exposed in HIV-1 envelope glycoproteins stabilized by flexible linkers as potent entry inhibitors and potential immunogens. Biochemistry. 2002;41:7176–82. doi: 10.1021/bi025646d. [DOI] [PubMed] [Google Scholar]
- 61.Srivastava IK, VanDorsten K, Vojtech L, Barnett SW, Stamatatos L. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J Virol. 2003;77:2310–20. doi: 10.1128/JVI.77.4.2310-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chakrabarti BK, Kong WP, Wu BY, et al. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol. 2002;76:5357–68. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grundner C, Mirzabekov T, Sodroski J, Wyatt R. Solid-phase proteoliposomes containing human immunodeficiency virus envelope glycoproteins. J Virol. 2002;76:3511–21. doi: 10.1128/JVI.76.7.3511-3521.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doan LX, Li M, Chen C, Yao Q. Virus-like particles as HIV-1 vaccines. Rev Med Virol. 2004;15:75–88. doi: 10.1002/rmv.449. [DOI] [PubMed] [Google Scholar]
- 65.Fouts T, Godfrey K, Bobb K, et al. Crosslinked HIV-1 envelope-CD4 receptor complexes elicit broadly cross-reactive neutralizing antibodies in rhesus macaques. Proc Natl Acad Sci USA. 2002;99:11842–7. doi: 10.1073/pnas.182412199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pantophlet R, Wilson IA, Burton DR. Hyperglycosylated mutants of human immunodeficiency virus (HIV) type 1 monomeric gp120 as novel antigens for HIV vaccine design. J Virol. 2003;77:5889–901. doi: 10.1128/JVI.77.10.5889-5901.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pantophlet R, Wilson IA, Burton DR. Improved design of an antigen with enhanced specificity for the broadly HIV-neutralizing antibody b12. Protein Eng Des Sel. 2004;17:749–58. doi: 10.1093/protein/gzh085. [DOI] [PubMed] [Google Scholar]
- 68.Brunel FM, Zwick MB, Cardoso RM, et al. Structure-function analysis of the epitope for 4E10, a broadly neutralizing human immunodeficiency virus type 1 antibody. J Virol. 2006;80:1680–7. doi: 10.1128/JVI.80.4.1680-1687.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou T, Xu L, Dey B, et al. Structural definition of a conserved neutralization epitope on HIV-1 gp120. Nature. 2007;445:732–37. doi: 10.1038/nature05580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80:1414–26. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tighe H, Takabayashi K, Schwartz D, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939–47. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 72.Wille-Reece U, Flynn BJ, Lore K, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in non-human primates. Proc Natl Acad Sci U S A. 2005;102:15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wille-Reece U, Wu CY, Flynn BJ, Kedl RM, Seder RA. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J Immunol. 2005;174:7676–83. doi: 10.4049/jimmunol.174.12.7676. [DOI] [PubMed] [Google Scholar]
- 74.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 75.Boggiano C, Manel N, Littman DR. Dendritic cell-mediated trans-enhancement of HIV-1 infectivity is independent of DC-SIGN. J Virol. 2006;82:2519–23. doi: 10.1128/JVI.01661-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boyd MR, Gustafson KR, McMahon JB, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–30. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Esser MT, Mori T, Mondor I, et al. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73:4360–71. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desrosiers RC, Wyand MS, Kodama T, et al. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989;86:6353–7. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphey-Corb M, Martin LN, Davison-Fairburn B, et al. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989;246:1293–7. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- 80.Gomez MB, Hildreth JE. Antibody to adhesion molecule LFA-1 enhances plasma neutralization of human immunodeficiency virus type 1. J Virol. 1995;69:4628–32. doi: 10.1128/jvi.69.8.4628-4632.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rizzuto CD, Sodroski JG. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–51. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Landau NR, Warton M, Littman DR. The envelope glyco-protein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature. 1988;334:159–62. doi: 10.1038/334159a0. [DOI] [PubMed] [Google Scholar]
- 83.Brighty DW, Rosenberg M, Chen IS, Ivey-Hoyle M. Envelope proteins from clinical isolates of human immunodeficiency virus type 1 that are refractory to neutralization by soluble CD4 possess high affinity for the CD4 receptor. Proc Natl Acad Sci U S A. 1991;88:7802–5. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daar ES, Ho DD. Relative resistance of primary HIV-1 isolates to neutralization by soluble CD4. Am J Med. 1991;90:22S–6. doi: 10.1016/0002-9343(91)90407-o. [DOI] [PubMed] [Google Scholar]
- 85.Allaway GP, Davis-Bruno KL, Beaudry GA, et al. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retro-viruses. 1995;11:533–9. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 86.Zhu P, Olson WC, Roux KH. Structural flexibility and functional valence of CD4-IgG2 (PRO 542): potential for cross-linking human immunodeficiency virus type 1 envelope spikes. J Virol. 2001;75:6682–6. doi: 10.1128/JVI.75.14.6682-6686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castagna A, Biswas P, Beretta A, Lazzarin A. The appealing story of HIV entry inhibitors: from discovery of biological mechanisms to drug development. Drugs. 2005;65:879–904. doi: 10.2165/00003495-200565070-00001. [DOI] [PubMed] [Google Scholar]
- 88.Gauduin MC, Allaway GP, Olson WC, Weir R, Maddon PJ, Koup RA. CD4-immunoglobulin G2 protects Hu-PBL-SCID mice against challenge by primary human immunodeficiency virus type 1 isolates. J Virol. 1998;72:3475–8. doi: 10.1128/jvi.72.4.3475-3478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacobson JM, Israel RJ, Lowy I, et al. Treatment of advanced human immunodeficiency virus type 1 disease with the viral entry inhibitor PRO 542. Antimicrob Agents Chemother. 2004;48:423–9. doi: 10.1128/AAC.48.2.423-429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Healey D, Dianda L, Moore JP, et al. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990;172:1233–42. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moore JP, Sattentau QJ, Klasse PJ, Burkly LC. A monoclonal antibody to CD4 domain 2 blocks soluble CD4-induced conformational changes in the envelope glycoproteins of human immunodeficiency virus type 1 (HIV-1) and HIV-1 infection of CD4+ cells. J Virol. 1992;66:4784–93. doi: 10.1128/jvi.66.8.4784-4793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhou T, Hamer DH, Hendrickson WA, Sattentau QJ, Kwong PD. Interfacial metal and antibody recognition. Proc Natl Acad Sci U S A. 2005;102:14575–80. doi: 10.1073/pnas.0507267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burkly L, Mulrey N, Blumenthal R, Dimitrov DS. Synergistic inhibition of human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion and infection by an antibody to CD4 domain 2 in combination with anti-gp120 antibodies. J Virol. 1995;69:4267–73. doi: 10.1128/jvi.69.7.4267-4273.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–77. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 95.Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16:100–3. doi: 10.1038/ng0597-100. [DOI] [PubMed] [Google Scholar]
- 96.Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–62. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 97.Samson M, Libert F, Doranz BJ, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–5. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 98.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–7. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 99.Samson M, Soularue P, Vassart G, Parmentier M. The genes encoding the human CC-chemokine receptors CC-CKR1 to CC-CKR5 (CMKBR1-CMKBR5) are clustered in the p21.3-p24 region of chromosome 3. Genomics. 1996;36:522–6. doi: 10.1006/geno.1996.0498. [DOI] [PubMed] [Google Scholar]
- 100.Zhou Y, Kurihara T, Ryseck RP, et al. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J Immunol. 1998;160:4018–25. [PubMed] [Google Scholar]
- 101.Lopalco L, Barassi C, Pastori C, et al. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 in vitro. J Immunol. 2000;164:3426–33. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- 102.Lopalco L, Pastori C, Cosma A, et al. Anti-cell antibodies in exposed seronegative individuals with HIV type 1-neutralizing activity. AIDS Res Hum Retroviruses. 2000;16:109–15. doi: 10.1089/088922200309458. [DOI] [PubMed] [Google Scholar]
- 103.Pastori C, Weiser B, Barassi C, et al. Long-lasting CCR5 internalization by antibodies in a subset of long-term nonprogressors: a possible protective effect against disease progression. Blood. 2006;107:4825–33. doi: 10.1182/blood-2005-06-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaheen F. Collman RG. Co-receptor antagonists as HIV-1 entry inhibitors. Curr Opin Infect Dis. 2004;17:7–16. doi: 10.1097/00001432-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 105.Dragic T, Trkola A, Thompson DA, et al. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc Natl Acad Sci U S A. 2000;97:5639–44. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baba M, Miyake H, Wang X, Okamoto M, Takashima K. Isolation and characterization of human immunodeficiency virus type 1 resistant to the small-molecule CCR5 antagonist TAK-652. Antimicrob Agents Chemother. 2007;51:707–15. doi: 10.1128/AAC.01079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Marozsan AJ, Kuhmann SE, Morgan T, et al. Generation and properties of a human immunodeficiency virus type 1 isolate resistant to the small molecule CCR5 inhibitor, SCH-417690 (SCH-D). Virology. 2005;338:182–99. doi: 10.1016/j.virol.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 108.Trkola A, Kuhmann SE, Strizki JM, et al. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc Natl Acad Sci U S A. 2002;99:395–400. doi: 10.1073/pnas.012519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuhmann SE, Platt EJ, Kozak SL, Kabat D. Cooperation of multiple CCR5 coreceptors is required for infections by human immunodeficiency virus type 1. J Virol. 2000;74:7005–15. doi: 10.1128/jvi.74.15.7005-7015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee B, Sharron M, Blanpain C, et al. Epitope mapping of CCR5 reveals multiple conformational states and distinct but overlapping structures involved in chemokine and coreceptor function. J Biol Chem. 1999;274:9617–26. doi: 10.1074/jbc.274.14.9617. [DOI] [PubMed] [Google Scholar]
- 111.Wu L, Gerard NP, Wyatt R, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–83. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 112.McKnight A, Wilkinson D, Simmons G, et al. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–6. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Strizki JM, Turner JD, Collman RG, Hoxie J, Gonzalez-Scar-ano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-1(89.6) but not the T-tropic isolate HIV-1(HxB). J Virol. 1997;71:5678–83. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leyland-Jones B. Trastuzumab: hopes and realities. Lancet Oncol. 2002;3:137–44. doi: 10.1016/s1470-2045(02)00676-9. [DOI] [PubMed] [Google Scholar]
- 115.Zuber B, Hinkula J, Levi M, Lundholm P, Nilsson C, Wahren B. Induction of immune responses and break of tolerance by DNA against the HIV-1 co-receptor CCR5 but no protections from SIV SM challenge. Virology. 2000;633:1–10. doi: 10.1006/viro.2000.0633. [DOI] [PubMed] [Google Scholar]
- 116.Chackerian B, Briglio L, Albert PS, Lowy DR, Schiller JT. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J Virol. 2004;78:4037–47. doi: 10.1128/JVI.78.8.4037-4047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khurana S, Kennedy M, King LR, Golding H. Identification of a linear peptide recognized by monoclonal antibody 2D7 capable of generating CCR5-specific antibodies with human immunodeficiency virus-neutralizing activity. J Virol. 2005;79:6791–800. doi: 10.1128/JVI.79.11.6791-6800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol. 2000;18:649–54. doi: 10.1038/76501. [DOI] [PubMed] [Google Scholar]
- 119.Navratilova I, Pancera M, Wyatt RT, Myszka DG. A biosensor-based approach toward purification and crystallization of G protein-coupled receptors. Anal Biochem. 2006;353:278–83. doi: 10.1016/j.ab.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 120.Navratilova I, Sodroski J, Myszka DG. Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal Biochem. 2005;339:271–81. doi: 10.1016/j.ab.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 121.Ji C, Brandt M, Dioszegi M, et al. Novel CCR5 monoclonal antibodies with potent and broad-spectrum anti-HIV activities. Antiviral Res. 2006;74:125–37. doi: 10.1016/j.antiviral.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 122.Steinberger P, Sutton JK, Rader C, Elia M, Barbas CF., III Generation and characterization of a recombinant human CCR5-specific antibody. A phage display approach for rabbit antibody humanization. J Biol Chem. 2000;275:36073–8. doi: 10.1074/jbc.M002765200. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Y, Pool C, Sadler K, et al. Selection of active ScFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry. 2004;43:12575–84. doi: 10.1021/bi0492152. [DOI] [PubMed] [Google Scholar]
- 124.Dalum I, Jensen MR, Hindersson P, Elsner HI, Mouritsen S. Breaking of B cell tolerance toward a highly conserved self protein. J Immunol. 1996;157:4796–804. [PubMed] [Google Scholar]
- 125.Tremblay MJ, Fortin JF, Cantin R. The acquisition of host-encoded proteins by nascent HIV-1. Immunol Today. 1998;19:346–51. doi: 10.1016/s0167-5699(98)01286-9. [DOI] [PubMed] [Google Scholar]
- 126.Lederman MM, Veazey RS, Offord R, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 127.Veazey RS, Klasse PJ, Schader SM, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 128.Hoxie JA, Fitzharris TP, Youngbar PR, Matthews DM, Rackowski JL, Radka SF. Nonrandom association of cellular antigens with HTLV-III virions. Hum Immunol. 1987;18:39–52. doi: 10.1016/0198-8859(87)90111-x. [DOI] [PubMed] [Google Scholar]
- 129.Lando Z, Sarin P, Megson M, et al. Association of human T-cell leukaemia/lymphoma virus with the Tac antigen marker for the human T-cell growth factor receptor. Nature. 1983;305:733–6. doi: 10.1038/305733a0. [DOI] [PubMed] [Google Scholar]
- 130.Young JA, Bates P, Willert K, Varmus HE. Efficient incorporation of human CD4 protein into avian leukosis virus particles. Science. 1990;250:1421–3. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- 131.Stott EJ, Chan WL, Mills KH, et al. Preliminary report: protection of cynomolgus macaques against simian immunodeficiency virus by fixed infected-cell vaccine. Lancet. 1990;336:1538–41. doi: 10.1016/0140-6736(90)93310-l. [DOI] [PubMed] [Google Scholar]
- 132.Stott EJ. Anti-cell antibody in macaques. Nature. 1991;353:393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- 133.Arthur LO, Bess JW, Jr, Sowder RC, II, et al. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–8. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 134.Esser MT, Graham DR, Coren LV, et al. Differential incorporation of CD45, CD80 (B7−1), CD86 (B7−2), and major histo-compatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J Virol. 2001;75:6173–82. doi: 10.1128/JVI.75.13.6173-6182.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Frank I, Stoiber H, Godar S, et al. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS. 1996;10:1611–20. doi: 10.1097/00002030-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 136.Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–65. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 137.Chan WL, Rodgers A, Hancock RD, et al. Protection in simian immunodeficiency virus-vaccinated monkeys correlates with anti-HLA class I antibody response. J Exp Med. 1992;176:1203–7. doi: 10.1084/jem.176.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arthur LO, Bess JW, Jr, Urban RG, et al. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J Virol. 1995;69:3117–24. doi: 10.1128/jvi.69.5.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stott JAN, Kent K, Kitchin P, et al. Biotechnology and AIDS. Instituto Polignafico; Rome: 1995. Viral and Cellular Antigens induce Protection Against Simian Immunodeficiency Virus Infection of Macaques. pp. 151–9. [Google Scholar]
- 140.Page M, Ojugo A, Imami N, Hardy G, Gotch F, Almond N. Specificity of anti-human leukocyte antigen antibody responses after immunization with Remune, an inactivated HIV-1 vaccine. AIDS. 2007;21:375–7. doi: 10.1097/QAD.0b013e328012b873. [DOI] [PubMed] [Google Scholar]
- 141.Bergmeier LA, Lehner T. Innate and adaptive mucosal immunity in protection against HIV infection. Adv Dent Res. 2006;19:21–8. doi: 10.1177/154407370601900106. [DOI] [PubMed] [Google Scholar]
- 142.MacDonald KS, Embree J, Njenga S, et al. Mother-child class I HLA concordance increases perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 1998;177:551–6. doi: 10.1086/514243. [DOI] [PubMed] [Google Scholar]
- 143.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–80. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 144.Dela Cruz CS, MacDonald KS, Barber BH. Anti-major histo-compatibility complex antibody responses in macaques via intradermal DNA immunizations. Vaccine. 2000;18:3152–65. doi: 10.1016/s0264-410x(00)00086-4. [DOI] [PubMed] [Google Scholar]
- 145.Lifson JD, Piatak M, Jr, Rossio JL, et al. Whole inactivated SIV virion vaccines with functional envelope glycoproteins: safety, immunogenicity, and activity against intrarectal challenge. J Med Primatol. 2002;31:205–16. doi: 10.1034/j.1600-0684.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- 146.Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 147.Kern F, LiPira G, Gratama JW, Manca F, Roederer M. Measuring Ag-specific immune responses: understanding immunopathogenesis and improving diagnostics in infectious disease, autoimmunity and cancer. Trends Immunol. 2005;26:477–84. doi: 10.1016/j.it.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 148.Eisenbarth GS, Nepom GT. Class II peptide multimers: promise for type 1A diabetes? Nat Immunol. 2002;3:344–5. doi: 10.1038/ni0402-344. [DOI] [PubMed] [Google Scholar]
- 149.McMichael AJ, Kelleher A. The arrival of HLA class II tetramers. J Clin Invest. 1999;104:1669–70. doi: 10.1172/JCI8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Moro M, Cecconi V, Martinoli C, et al. Generation of functional HLA-DR*1101 tetramers receptive for loading with pathogen-or tumour-derived synthetic peptides. BMC Immunol. 2005;6:24. doi: 10.1186/1471-2172-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–6. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 152.Wei X, Ghosh SK, Taylor ME, et al. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–22. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 153.Bounou S, Leclerc JE, Tremblay MJ. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4(+)-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J Virol. 2002;76:1004–14. doi: 10.1128/JVI.76.3.1004-1014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fortin JF, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–96. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


