Abstract
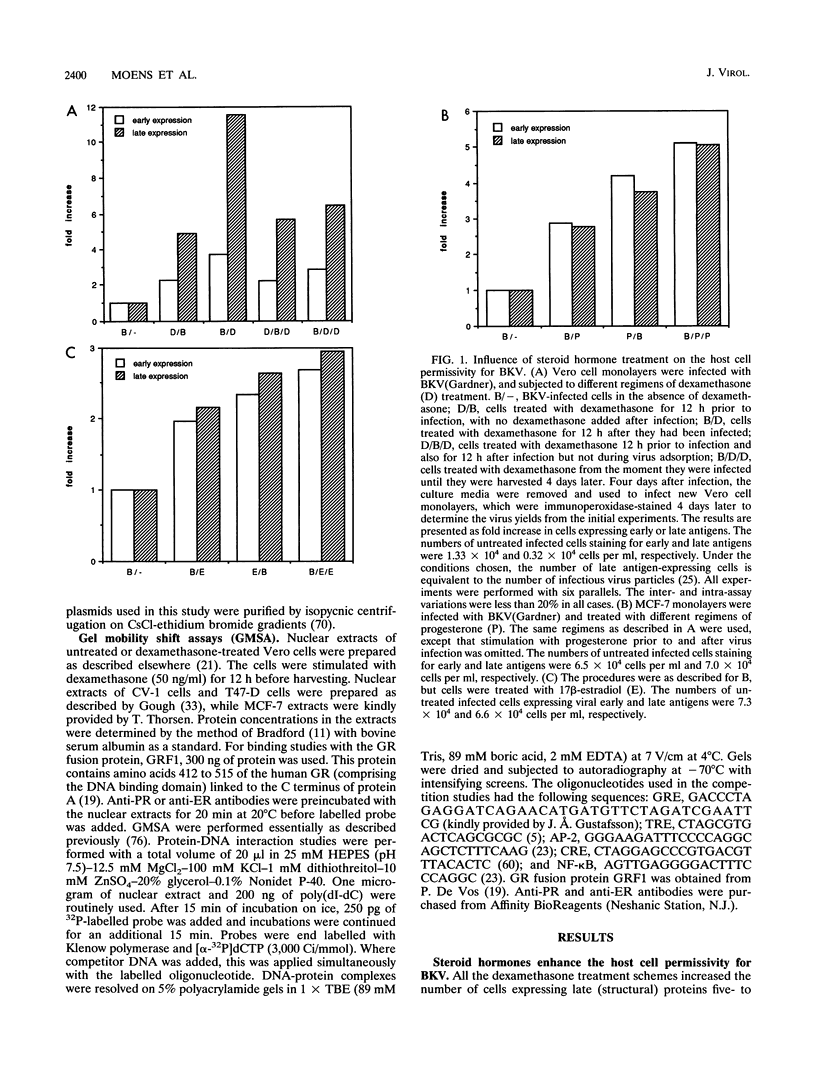
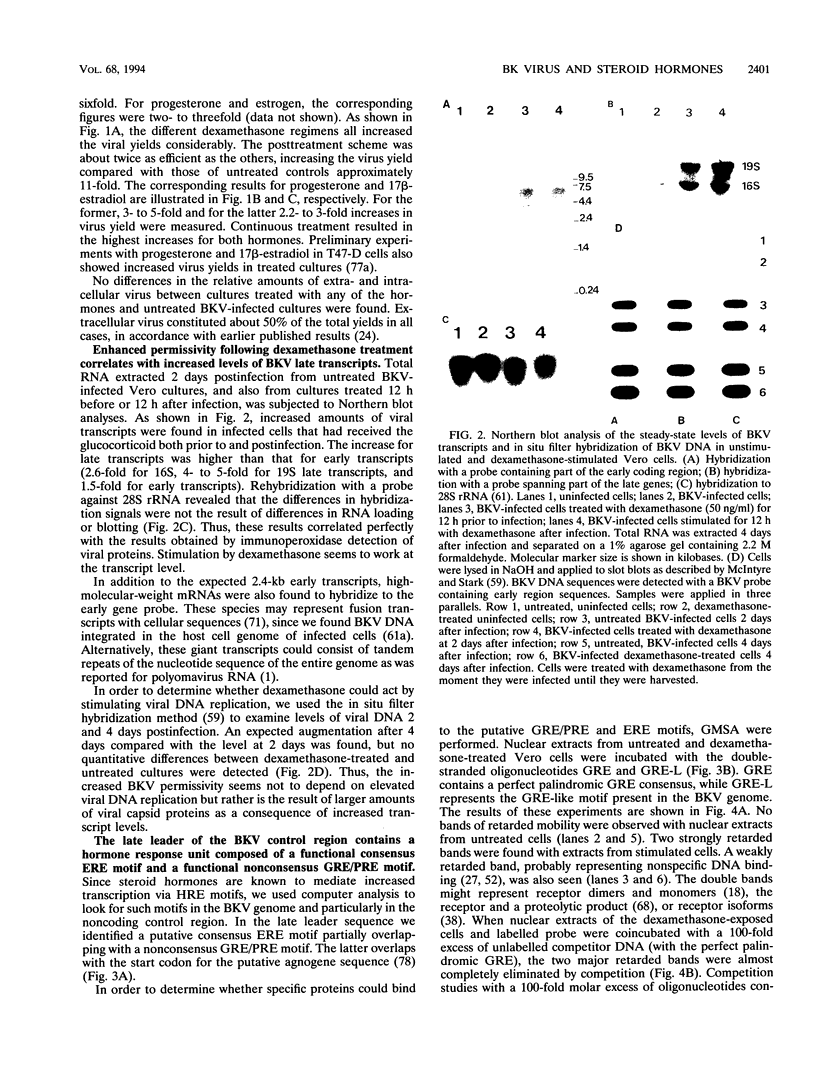
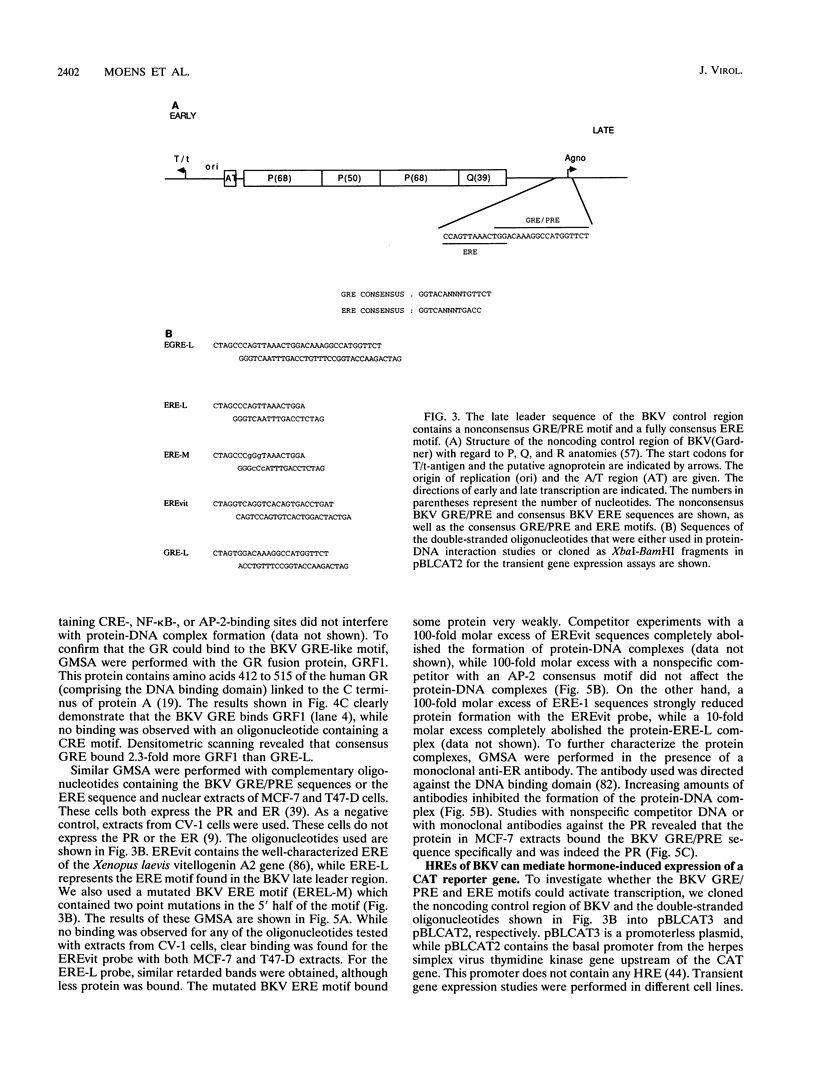
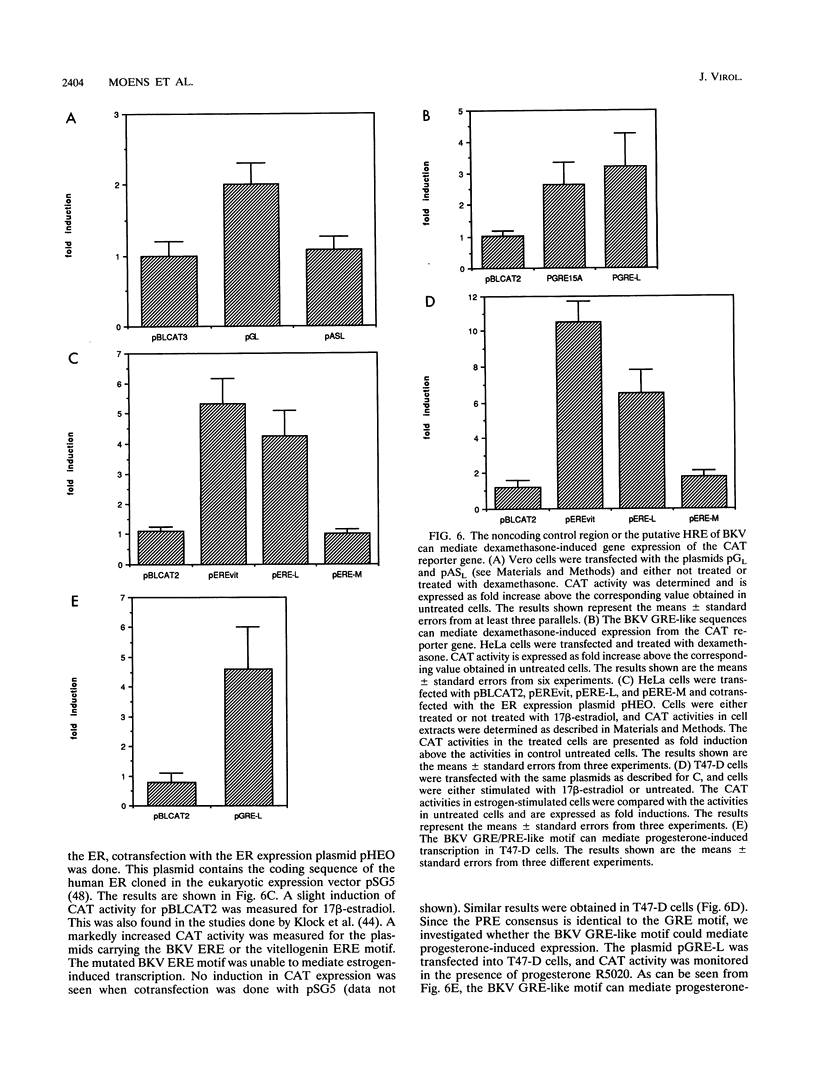
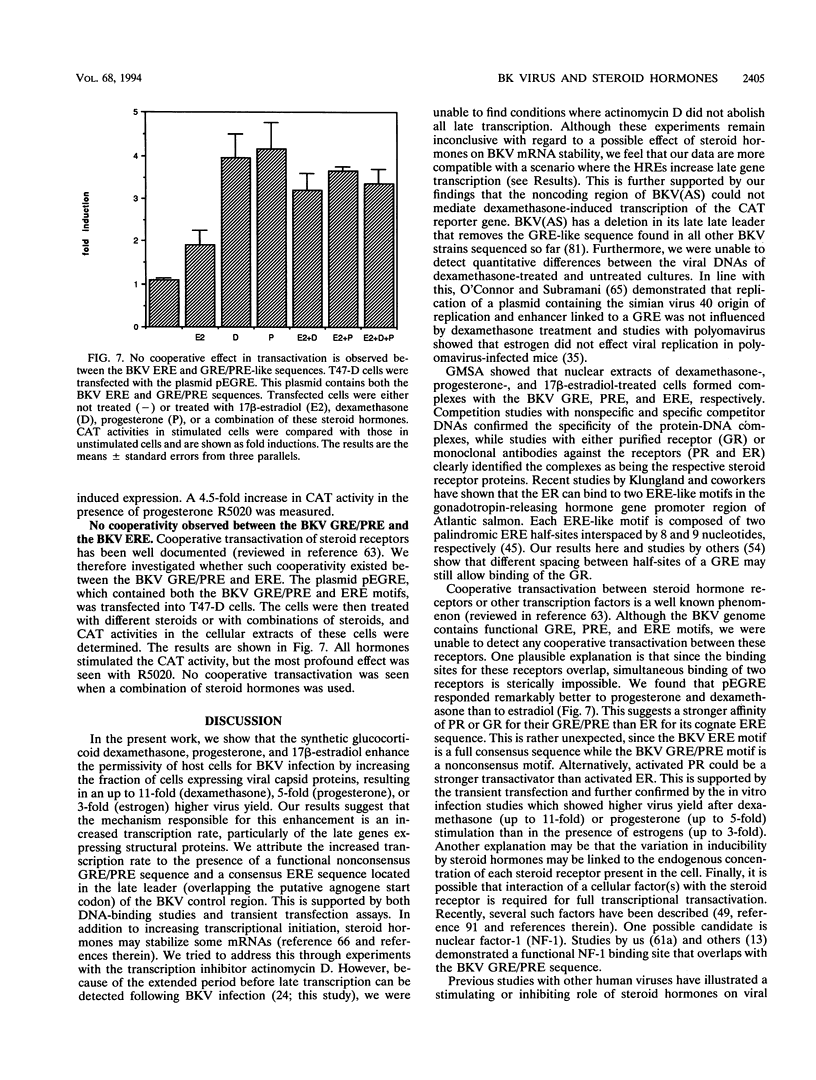
The effect of steroid hormones on multiplication of the human polyomavirus BK (BKV) was studied. Physiological concentrations of the synthetic glucocorticoid dexamethasone, progesterone R5020, or estrogen 17 beta-estradiol enhanced the permissivity of the host cell for BKV, resulting in an up to 11-fold (dexamethasone), 5-fold (progesterone), or 3-fold (17 beta-estradiol) higher virus yield. The increase in virus yield in dexamethasone-stimulated cells correlated with enhanced steady-state levels of viral transcripts. The late leader sequence of the BKV control region contains a hormone response unit composed of a nonconsensus glucocorticoid and/or progesterone response element (GRE/PRE) and a fully consensus estrogen response element (ERE). DNA-protein binding studies showed that the glucocorticoid receptor and the progesterone receptor bound to this BKV GRE/PRE-like sequence, while the estrogen receptor could bind to the BKV ERE motif. By transient transfection assays, we were able to show that these sequences can mediate steroid hormone-induced gene expression. However, no cooperative transactivation effect between the BKV GRE/PRE-like motif and BKV ERE motif was observed. This BKV hormone response unit may play an important role in vivo by enhancing a productive BKV infection, and perhaps also by reactivating a latent infection, during physiological or pathological conditions accompanied by increased steroid hormone levels.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Polyoma virus giant RNAs contain tandem repeats of the nucleotide sequence of the entire viral genome. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4754–4758. doi: 10.1073/pnas.75.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Amstey M. S. Effect of pregnancy hormones on herpesvirus and other deoxyribonucleic acid viruses. Am J Obstet Gynecol. 1977 Sep 15;129(2):159–163. doi: 10.1016/0002-9378(77)90738-4. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Baniahmad C., Muller M., Altschmied J., Renkawitz R. Co-operative binding of the glucocorticoid receptor DNA binding domain is one of at least two mechanisms for synergism. J Mol Biol. 1991 Nov 20;222(2):155–165. doi: 10.1016/0022-2836(91)90202-h. [DOI] [PubMed] [Google Scholar]
- Bauer G. Induction of Epstein-Barr virus early antigens by corticosteroids: inhibition by TPA and retinoic acid. Int J Cancer. 1983 Mar 15;31(3):291–295. doi: 10.1002/ijc.2910310307. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beato M. Induction of transcription by steroid hormones. Biochim Biophys Acta. 1987 Nov 20;910(2):95–102. doi: 10.1016/0167-4781(87)90060-1. [DOI] [PubMed] [Google Scholar]
- Bocquel M. T., Kumar V., Stricker C., Chambon P., Gronemeyer H. The contribution of the N- and C-terminal regions of steroid receptors to activation of transcription is both receptor and cell-specific. Nucleic Acids Res. 1989 Apr 11;17(7):2581–2595. doi: 10.1093/nar/17.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown P., Tsai T., Gajdusek D. C. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am J Epidemiol. 1975 Oct;102(4):331–340. doi: 10.1093/oxfordjournals.aje.a112169. [DOI] [PubMed] [Google Scholar]
- Chakraborty T., Das G. C. Proteins of the nuclear factor-1 family act as an activator of the late promoter in human polyomavirus BK in vitro. J Gen Virol. 1991 Aug;72(Pt 8):1935–1942. doi: 10.1099/0022-1317-72-8-1935. [DOI] [PubMed] [Google Scholar]
- Chesters P. M., Heritage J., McCance D. J. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983 Apr;147(4):676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- Coleman D. V., Daniel R. A., Gardner S. D., Field A. M., Gibson P. E. Polyoma virus in urine during pregnancy. Lancet. 1977 Oct 1;2(8040):709–710. doi: 10.1016/s0140-6736(77)90514-1. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- De Vos P., Claessens F., Peeters B., Rombauts W., Heyns W., Verhoeven G. Interaction of androgen and glucocorticoid receptor DNA-binding domains with their response elements. Mol Cell Endocrinol. 1993 Jan;90(2):R11–R16. doi: 10.1016/0303-7207(93)90160-l. [DOI] [PubMed] [Google Scholar]
- DeMarzo A. M., Beck C. A., Onate S. A., Edwards D. P. Dimerization of mammalian progesterone receptors occurs in the absence of DNA and is related to the release of the 90-kDa heat shock protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):72–76. doi: 10.1073/pnas.88.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei R., Marmo F., Corte D., Sampietro M. G., Franceschini E., Urbano P. Age-related changes in the prevalence of precipitating antibodies to BK virus in infants and children. J Med Microbiol. 1982 Aug;15(3):285–291. doi: 10.1099/00222615-15-3-285. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Trifiro M. A., Plante R. K., Nemer M., Eriksson P., Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol. 1989 Dec;9(12):5305–5314. doi: 10.1128/mcb.9.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faisst S., Meyer S. Compilation of vertebrate-encoded transcription factors. Nucleic Acids Res. 1992 Jan 11;20(1):3–26. doi: 10.1093/nar/20.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaegstad T., Traavik T. BK virus in cell culture: infectivity quantitation and sequential expression of antigens detected by immunoperoxidase staining. J Virol Methods. 1987 May;16(1-2):139–146. doi: 10.1016/0166-0934(87)90038-3. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Traavik T., Christie K. E., Joergensen J. Neutralization test for BK virus: plaque reduction detected by immunoperoxidase staining. J Med Virol. 1986 Jul;19(3):287–296. doi: 10.1002/jmv.1890190311. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., MacKenzie E. F., Smith C., Porter A. A. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984 May;37(5):578–586. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaub M. P., Bellard M., Scheuer I., Chambon P., Sassone-Corsi P. Activation of the ovalbumin gene by the estrogen receptor involves the fos-jun complex. Cell. 1990 Dec 21;63(6):1267–1276. doi: 10.1016/0092-8674(90)90422-b. [DOI] [PubMed] [Google Scholar]
- Ghosh D. Glucocorticoid receptor-binding site in the human immunodeficiency virus long terminal repeat. J Virol. 1992 Jan;66(1):586–590. doi: 10.1128/jvi.66.1.586-590.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. E., Field A. M., Gardner S. D., Coleman D. V. Occurrence of IgM antibodies against BK and JC polyomaviruses during pregnancy. J Clin Pathol. 1981 Jun;34(6):674–679. doi: 10.1136/jcp.34.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Bernard H. U., Seedorf K., Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987 Dec 1;6(12):3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M. Rapid and quantitative preparation of cytoplasmic RNA from small numbers of cells. Anal Biochem. 1988 Aug 15;173(1):93–95. doi: 10.1016/0003-2697(88)90164-9. [DOI] [PubMed] [Google Scholar]
- Heritage J., Chesters P. M., McCance D. J. The persistence of papovavirus BK DNA sequences in normal human renal tissue. J Med Virol. 1981;8(2):143–150. doi: 10.1002/jmv.1890080208. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Weinberger C., Ong E. S., Cerelli G., Oro A., Lebo R., Thompson E. B., Rosenfeld M. G., Evans R. M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985 Dec 19;318(6047):635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Hyder S. M., Stancel G. M., Nawaz Z., McDonnell D. P., Loose-Mitchell D. S. Identification of an estrogen response element in the 3'-flanking region of the murine c-fos protooncogene. J Biol Chem. 1992 Sep 5;267(25):18047–18054. [PubMed] [Google Scholar]
- Härd T., Dahlman K., Carlstedt-Duke J., Gustafsson J. A., Rigler R. Cooperativity and specificity in the interactions between DNA and the glucocorticoid receptor DNA-binding domain. Biochemistry. 1990 Jun 5;29(22):5358–5364. doi: 10.1021/bi00474a022. [DOI] [PubMed] [Google Scholar]
- James C. B., Vanderpool E. A., Roane P. Acceleration of adenovirus replication and increased virion production by treatment with the steroid hormone 17 beta-estradiol. Microbiol Immunol. 1992;36(1):99–103. doi: 10.1111/j.1348-0421.1992.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Katsanakis C. D., Sekeris C. E., Spandidos D. A. The human immunodeficiency virus long terminal repeat contains sequences showing partial homology to glucocorticoid responsive elements. Anticancer Res. 1991 Jan-Feb;11(1):381–383. [PubMed] [Google Scholar]
- Klock G., Strähle U., Schütz G. Oestrogen and glucocorticoid responsive elements are closely related but distinct. Nature. 1987 Oct 22;329(6141):734–736. doi: 10.1038/329734a0. [DOI] [PubMed] [Google Scholar]
- Klungland H., Andersen O., Kisen G., Aleström P., Tora L. Estrogen receptor binds to the salmon GnRH gene in a region with long palindromic sequences. Mol Cell Endocrinol. 1993 Sep;95(1-2):147–154. doi: 10.1016/0303-7207(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Koment R. W. Lytic cytomegalovirus replication and the hormones of human pregnancy. J Med Virol. 1985 Feb;15(2):149–156. doi: 10.1002/jmv.1890150207. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Kupfer S. R., Marschke K. B., Wilson E. M., French F. S. Receptor accessory factor enhances specific DNA binding of androgen and glucocorticoid receptors. J Biol Chem. 1993 Aug 15;268(23):17519–17527. [PubMed] [Google Scholar]
- Kupfer S. R., Summers W. C. Identification of a glucocorticoid-responsive element in Epstein-Barr virus. J Virol. 1990 May;64(5):1984–1990. doi: 10.1128/jvi.64.5.1984-1990.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Shaw Y. T., Chiou S. T., Chang W. C., Lai M. D. The effects of glucocorticoid hormone on the expression of c-jun. FEBS Lett. 1991 Mar 11;280(1):134–136. doi: 10.1016/0014-5793(91)80221-n. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Markham P. D., Salahuddin S. Z., Veren K., Orndorff S., Gallo R. C. Hydrocortisone and some other hormones enhance the expression of HTLV-III. Int J Cancer. 1986 Jan 15;37(1):67–72. doi: 10.1002/ijc.2910370112. [DOI] [PubMed] [Google Scholar]
- Markowitz R. B., Dynan W. S. Binding of cellular proteins to the regulatory region of BK virus DNA. J Virol. 1988 Sep;62(9):3388–3398. doi: 10.1128/jvi.62.9.3388-3398.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P., Stark G. R. A quantitative method for analyzing specific DNA sequences directly from whole cells. Anal Biochem. 1988 Oct;174(1):209–214. doi: 10.1016/0003-2697(88)90537-4. [DOI] [PubMed] [Google Scholar]
- Moens U., Subramaniam N., Johansen B., Aarbakke J. The c-fos cAMP-response element: regulation of gene expression by a beta 2-adrenergic agonist, serum and DNA methylation. Biochim Biophys Acta. 1993 Apr 29;1173(1):63–70. doi: 10.1016/0167-4781(93)90243-7. [DOI] [PubMed] [Google Scholar]
- Moens U., Sundsfjord A., Flaegstad T., Traavik T. BK virus early RNA transcripts in stably transformed cells: enhanced levels induced by dibutyryl cyclic AMP, forskolin and 12-O-tetradecanoylphorbol-13-acetate treatment. J Gen Virol. 1990 Jul;71(Pt 7):1461–1471. doi: 10.1099/0022-1317-71-7-1461. [DOI] [PubMed] [Google Scholar]
- Notter M. F., Docherty J. J. Steroid hormone alteration of herpes simplex virus type 1 replication. J Med Virol. 1978;2(3):247–252. doi: 10.1002/jmv.1890020308. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Subramani S. Do transcriptional enhancers also augment DNA replication? Nucleic Acids Res. 1988 Dec 9;16(23):11207–11222. doi: 10.1093/nar/16.23.11207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., LaForge K. S., Sehgal P. B. On the mechanism for efficient repression of the interleukin-6 promoter by glucocorticoids: enhancer, TATA box, and RNA start site (Inr motif) occlusion. Mol Cell Biol. 1990 Nov;10(11):5736–5746. doi: 10.1128/mcb.10.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster C., Chasserot-Golaz S., Urier G., Beck G., Sergeant A. Evidence for a functional glucocorticoid responsive element in the Epstein-Barr virus genome. Mol Endocrinol. 1991 Feb;5(2):267–272. doi: 10.1210/mend-5-2-267. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Shah K., Daniel R., Madden D., Stagno S. Serological investigation of BK papovavirus infection in pregnant women and their offspring. Infect Immun. 1980 Oct;30(1):29–35. doi: 10.1128/iai.30.1.29-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Strömstedt P. E., Poellinger L., Gustafsson J. A., Carlstedt-Duke J. The glucocorticoid receptor binds to a sequence overlapping the TATA box of the human osteocalcin promoter: a potential mechanism for negative regulation. Mol Cell Biol. 1991 Jun;11(6):3379–3383. doi: 10.1128/mcb.11.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundsfjord A., Johansen T., Flaegstad T., Moens U., Villand P., Subramani S., Traavik T. At least two types of control regions can be found among naturally occurring BK virus strains. J Virol. 1990 Aug;64(8):3864–3871. doi: 10.1128/jvi.64.8.3864-3871.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Ogura T., Kamiya S., Sato H., Yoshie T., Ogura H., Hatano M. Enhanced replication of human cytomegalovirus in human fibroblasts treated with dexamethasone. J Gen Virol. 1984 Oct;65(Pt 10):1759–1767. doi: 10.1099/0022-1317-65-10-1759. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Ogura T., Kamiya S., Yoshie T., Yabuki Y., Hatano M. Dexamethasone enhances human cytomegalovirus replication in human epithelial cell cultures. Virology. 1984 Jul 30;136(2):448–452. doi: 10.1016/0042-6822(84)90182-x. [DOI] [PubMed] [Google Scholar]
- Tavis J. E., Walker D. L., Gardner S. D., Frisque R. J. Nucleotide sequence of the human polyomavirus AS virus, an antigenic variant of BK virus. J Virol. 1989 Feb;63(2):901–911. doi: 10.1128/jvi.63.2.901-911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A. M., Ettinger R., Kim N., Marshak-Rothstein A., Wotiz H. H. Development and characterization of monoclonal antibodies to a specific domain of human estrogen receptor. Steroids. 1990 May;55(5):196–208. doi: 10.1016/0039-128x(90)90017-6. [DOI] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Tur-Kaspa R., Burk R. D., Shaul Y., Shafritz D. A. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1627–1631. doi: 10.1073/pnas.83.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tur-Kaspa R., Laub O. Corticosteroids stimulate hepatitis B virus DNA, mRNA and protein production in a stable expression system. J Hepatol. 1990 Jul;11(1):34–36. doi: 10.1016/0168-8278(90)90268-v. [DOI] [PubMed] [Google Scholar]
- Wahli W., Martinez E., Corthésy B., Cardinaux J. R. cis- and trans-acting elements of the estrogen-regulated vitellogenin gene B1 of Xenopus laevis. J Steroid Biochem. 1989;34(1-6):17–32. doi: 10.1016/0022-4731(89)90062-9. [DOI] [PubMed] [Google Scholar]
- Weisz A., Cicatiello L., Persico E., Scalona M., Bresciani F. Estrogen stimulates transcription of c-jun protooncogene. Mol Endocrinol. 1990 Jul;4(7):1041–1050. doi: 10.1210/mend-4-7-1041. [DOI] [PubMed] [Google Scholar]
- Weisz A., Rosales R. Identification of an estrogen response element upstream of the human c-fos gene that binds the estrogen receptor and the AP-1 transcription factor. Nucleic Acids Res. 1990 Sep 11;18(17):5097–5106. doi: 10.1093/nar/18.17.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yoshinaga S. K., Peterson C. L., Herskowitz I., Yamamoto K. R. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science. 1992 Dec 4;258(5088):1598–1604. doi: 10.1126/science.1360703. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Dong J. M., Chiu J. F. Regulation of alpha-fetoprotein gene expression by antagonism between AP-1 and the glucocorticoid receptor at their overlapping binding site. J Biol Chem. 1991 May 5;266(13):8248–8254. [PubMed] [Google Scholar]