Abstract
We have prepared a caged form (MRS2703) of a potent dual agonist of the P2Y1 and P2Y12 nucleotide receptors, 2-MeSADP, by blocking the β-phosphate group with a 1-(3,4-dimethyloxyphenyl)eth-1-yl phosphoester. Although MRS2703 is itself inactive at human P2Y1 and P2Y12 receptors expressed heterologously in 1321N1 astrocytoma cells or in washed human platelets, this derivative readily regenerates the parent agonist upon mild irradiation with long-wave UV light (360 nm). The functional effect of the regenerated agonist was demonstrated by a rise in intracellular calcium mediated by either P2Y1 or P2Y12 receptors in transfected cells. Washed human platelets exposed to a solution of MRS2703 were induced to aggregate upon UV irradiation. At 1.0 μM MRS2703, full aggregation was achieved within one minute of irradiation. Thus, this caged nucleotide promises to be a useful probe for potent P2Y receptor activation with light-directed spatial and temporal control.
Keywords: aggregation, nucleotides, G protein-coupled receptors, phospholipase C, calcium
1. Introduction
Caged molecules, in which light is able to rapidly and site-specifically generate a reactive or biologically-active chemical species, have been designed based on the photocleavage of the 2-nitrobenzyl protecting group. The 2-nitrobenzyl group was originally introduced as an orthogonal photoremovable protecting group for use in peptide synthesis by Patchornik et al. [1]. Since then various chemically improved modifications of the photolabile 2-nitrobenzyl group, such as the 1-(3,4-dimethyloxyphenyl)eth-1-yl group, have been utilized [2]. The term ‘caged’ was first coined by Kaplan et al. [19,20], and caged ATP was the first to be introduced to study organized biological systems. A caged-ATP derivative (P3-1-(2-nitrophenylethyladenosine 5′-triphosphate) has been used to probe the fast kinetics of muscle contraction, etc. [9]. This principle of photocleavage has also been effectively applied to selective deprotection for synthesis in a spatially addressable fashion on a surface array [3], masking of intracellular and extracellular signaling molecules for biological studies [4-6], and the light-reversible rapid activation of proteins [7,8]. In the area of platelet biology, an oligonucleotide aptamer consisting of a ssDNA 15mer was caged, by preparation of a thymidine 4-O-(2-nitrophenylethyl) derivative, to provide a UV-light activatable derivative [10]. This derivative when unmasked with light bound to and inactivated R-thrombin, which is a key player in the blood-clotting cascade. Recently, coumarin-based photosensitive protecting groups have been applied to cage biologically-active molecules [11].
The P2Y1 and P2Y12 receptors are G protein-coupled receptors of the rhodopsin family present on the surface of platelets that respond to extracellular ADP to induce aggregation [12-14]. This activation is an essential step in the process of platelet aggregation in vivo. In in vitro studies of platelet function, the activation of both receptors by ADP is required for complete aggregation. Thus, application of a selective antagonist of either the P2Y1 or the P2Y12 receptor inhibits aggregation. A potent and selective P2Y1 receptor nucleotide antagonist MRS2500, in which the ribose moiety is conformationally constrained to “fit” the preferred geometry of the binding site, inhibited both aggregation and the initial shape change in platelets, which are characteristic of the effects of ADP. MRS2500 was also shown to act as a P2Y1 receptor antagonist in vivo to impede systemic thromboembolism and localized arterial thrombosis [14,15].
We previously applied the 2-nitrobenzyl group in the form of a nucleoside 5′-ether to prepare a caged agonist of the A1 adenosine receptor [16]. In the present study we have synthesized and characterized pharmacologically a 2-nitrobenzyl-type derivative of the potent proaggregatory receptor probe, 2-methylthio-ADP (2-MeSADP), which was shown to activate both P2Y1 and P2Y12 receptors on the surface of platelets. 2-MeSADP is approximately three orders of magnitude more potent than ADP as an agonist of these two P2Y receptor subtypes and displays nanomolar binding affinity at each. Structure-activity studies have been extensively reviewed [12]. This caged nucleotide allows spatial and temporal control over the proaggregatory signal, as demonstrated in in vitro models.
2. Materials and methods
2.1. Materials
The 1321N1 astrocytoma cells stably transfected with human P2Y1 and P2Y12 receptors were generously provided by Prof. T. K. Harden (University of North Carolina, Chapel Hill, NC). Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum (FBS) were purchased from Life Technologies (Rockville, MD). Plastic collagen-coated cellware was purchased from Becton Dickinson (Bedford, MA). Calcium Mobilization Assay Kit was purchased from Molecular Devices (Sunnyvale, CA).
1H NMR spectra were obtained with a Varian Gemini 300 spectrometer using D2O as a solvent. The chemical shifts are expressed as relative ppm from HOD (4.80 ppm). 31P NMR spectra were recorded at room temperature by use of Varian XL 300 spectrometer (121.42 MHz); orthophosphoric acid (85%) was used as an external standard. Purity of compounds was checked using a Hewlett–Packard 1100 HPLC equipped with a Zorbax Eclipse 5-μm XDB-C18 analytical column (250 X 4.6 mm; Agilent Technologies Inc, Palo Alto, CA). System A: linear gradient solvent system: 5 mM TBAP (tetrabutylammonium dihydrogenphosphate)-CH3CN from 80:20 to 40:60 in 20 min; the flow rate was 1 ml/min. Peaks were detected by UV absorption with a diode array detector. High-resolution mass measurements were performed on Micromass/Waters LCT Premier Electrospray Time of Flight (TOF) mass spectrometer coupled with a Waters HPLC system. Purification of the caged nucleotide was carried out by HPLC using a Luna 5μ RP-C18(2) semipreparative column (250 × 4.6 mm; Phenomenex, Torrance, CA). System B: 10 mM TEAA(triethylammonium acetate)-CH3CN from 90:10 to 60:40 in 30 min; the flow rate was 2 mL/min.
1-(4,5-Dimethoxy-2-nitrophenyl) diazoethane Generation Kit was purchased from Invitrogen Molecular Probes (Eugene, OR). 2-MeSADP (1) was purchased from Sigma-Aldrich (St. Louis, MO) and other reagents and solvents were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Chemical synthesis
P1-2-(Methylthio)adenosyl-P2-(RS)-1-(4,5-dimethoxy-2-nitrophenyl)ethyl pyrophosphate, Triethylammonium Salt (4)
To prepare the reactive diazoethane (3) a solution of 4,5-dimethoxy-2-nitroacetophenone hydrazone (2, 25 mg, 0.10 mmol) in DMF (1 mL) was treated with MnO2 (100 mg, 1.15 mmol). After stirring for 30 min at room temperature, the reaction mixture was filtered through Celite® diatomaceous earth (100 mg) contained in a Pasteur pipette to obtain a red solution that contains the 1-(4,5-dimethoxy-2-nitrophenyl) diazoethane (3). This solution was used in the next step without further purification. To a solution of 2-MeSADP (1, 5 mg, 0.008 mmol) in H2O (1 mL) was added the red solution that contains the 1-(4,5-dimethoxy-2-nitrophenyl)-diazoethane (3). To avoid light the reaction flasks are covered with aluminum foil. The reaction mixture was stirred at room temperature for 24 h. The aqueous phase was treated with CH2Cl2. After removal of the solvent, the residue was purified by semipreparative HPLC as described above to obtain the mixture of diastereoisomers 4ab (4.8 mg, 66%) as a white solid. 1H NMR (D2O) δ 8.05 (s, 1H), 7.97 (s, 1H), 7.44 (s, 1H), 7.39 (s, 1H), 7.32 (s, 1H), 7.19 (s, 1H), 6.04 (m, 2H), 5.90 (d, J = 4.8 Hz, 1H), 5.82 (d, J = 4.8 Hz, 1H), 4.50 (m, 1H), 4.41 (m, 1H), 4.33 (m, 1H), 4.30 (m, 1H), 4.21 (m, 1H), 4.13 (m, 3H), 3.99 (m, 2H), 3.94 (s, 1H), 3.91 (s, 1H), 3.71 (s, 1H), 3.68 (s, 1H), 2.56 (s, 1H), 2.54 (s, 1H), 1.50 (d, J = 6.3 Hz, 6H); 31P NMR (D2O) δ -11.17 (d, J = 20.8 Hz), -11.33 (d, J = 18.3 Hz), -11.82 (d, J = 20.8 Hz), -12.18 (d, J = 18.9 Hz); HRMS m/z found 681.0742 (M – H+)-. C21H27N6O14P2S requires 681.0781; HPLC (system A) 15.2 min.
HPLC analysis using System A was carried out on a solution of MRS2703 (50 μM in PBS) before and after irradiation (as described in Section 2.4) for 2 min. Following this irradiation all of the MRS2703, having a retention time (r.t.) of 15.2 min, had disappeared and the major peak observed was a new peak with the identical r.t. as the parent 2-MeSADP (12.9 min).
2.3. Cell culture
Human 1321N1 astrocytoma cells stably transfected with the human P2Y1 or P2Y12 receptors were grown at 37°C in a humidified incubator with 5% CO2/95% air in DMEM/F-12 medium (1:1) supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 2 mM L-glutamine. The cells were passaged using trypsinization every 4-5 days.
2.4. Calcium mobilization assay
Cells were grown overnight in 100 μl of medium in 96-well flat-bottom plates at 37°C at 5% CO2 or until they reached ∼60-80% confluency. The calcium assay kit (Molecular Devices, Sunnyvale. CA, USA) was used as directed with no washing of cells and with probenecid added to the loading dye at a final concentration of 2.5 mM to increase dye retention. Cells were loaded with 50 μl dye with probenecid in each well and incubated for 45 min at room temperature. The compound plate was prepared with dilutions of various compounds in Hank's Buffer at pH 7.2. For irradiation of the caged agonist, a solution of MRS2703 in PBS was exposed to UV light under various conditions of wavelengths and for a range of times. A UV light (Spectroline model, UV-400; Spectronics Co., Westbury, USA) was outfitted with a 360 nm filter (Edmund Industrial Optics, Barrington, NJ, USA), and a PBS solution of MRS2703 (20 μM) in a glass cuvette was irradiated at a distance of 1.0 cm. Room lighting was dimmed during the experiments using MRS2703 under nonirradiated conditions. Samples were run in duplicate with a Flexstation I (Molecular Devices) at room temperature. Cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to a compound. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure.
2.5. Preparation of human washed platelets
Human washed platelets were prepared as previously described [22]. Briefly, fresh blood obtained from healthy donors was centrifuged at 175×g for 15 min at 37°C, platelet rich plasma was removed, and centrifuged at 1570×g for 15 min at 37°C. The platelet pellet was washed twice in Tyrode's buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 5.5 mM glucose, 5 mM Hepes, pH 7.3) containing 0.35% human serum albumin, and finally resuspended at a density of 3×105 platelets/μl in the same buffer in the presence of 0.02 U/ml of the ADP scavenger apyrase (adenosine 5′-triphosphate diphosphohydrolase, EC 3.6.1.5), a concentration sufficient to prevent desensitization of platelet ADP receptors during storage. Platelets were kept at 37°C throughout all experiments.
2.6. Platelet aggregation studies
Aggregation was measured at 37°C by a turbidimetric method in a dual-channel Payton aggregometer (Payton Associates, Scarborough, Ontario, Canada). A solution of MRS2703 in PBS was exposed to UV light (12 W light source, with a peak UV wavelength of 254 nm) for various time periods and immediately added to a 450 μl aliquot of platelet suspension stirred at 1100 rpm, in the presence of human fibrinogen (0.8 mg/ml), in a final volume of 500 μl.
2.7. Statistical analysis
Pharmacological parameters were analyzed with the GraphPAD Prism software (version 4.0, GraphPAD Prism, San Diego, CA). Data were expressed as mean ± standard error. Statistical significance was calculated using the Student's t-test. P values less than 0.05 (P<0.05) were considered to be statistically significant.
3. Results
3.1. Design of reagent and chemical synthesis
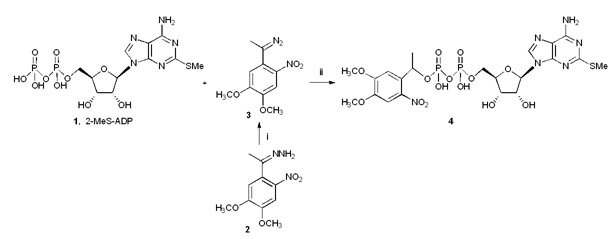
The caged nucleotide MRS2703 (4) was prepared from 2-MeSADP (1) and the in situ generated phenyl diazoethane as shown in Figure 1 [2]. The presence of the veratryl group is intended to allow unmasking by light of longer wavelength than is required for simple 2-nitrobenzyl derivatives. The presence of the ethyl moiety reduces side reactions as a result of the generation of photoproducts, which in this case would be an acetylaryl compound that is less reactive than the corresponding benzaldehyde. HPLC analysis indicated a nearly complete conversion of a dilute solution of MRS2703 to the parent 2-MeSADP during a 2 min irradiation.
Figure 1.
Structure and preparation of caged nucleotide MRS2703 4. The caged nucleotide could be reconverted to the starting material, agonist 2-MeSADP 1 upon irradiation. Reagents and conditions: (i) MnO2, DMF, r.t. (ii) H2O, rt.
3.2. Characterization in astrocytoma cells
The photoregeneration of the potent P2Y receptor agonist 2-MeSADP was first tested using the recombinant human P2Y1 receptor expressed in 1321N1 astrocytoma cells (Figure 2). In a control experiment these cells were exposed to 2-MeSADP, which induced a concentration-dependent rise in [Ca2+]i levels, with an EC50 value of 2.03 ± 0.35 nM (n=3), similar to previously reported values [18]. MRS2703, without exposure to UV light, was weak in the stimulation of calcium mobilization, with an EC50 value estimated to be ≥ 10,000 nM (Fig. 2).
Figure 2.
The ability of 2-MeSADP, and the caged 2-MeSADP (MRS2703) to activate the human P2Y1 receptor stably expressed in 1321N1 astrocytoma cells. Cells were loaded with 50 μl dye with 2.5 mM probenecid in each well and incubated for 45 min at room temperature. The agonists were tested without exposure to UV light. 2-MeSADP induced a concentration-dependent rise in [Ca2+]i levels, with an EC50 value of 2.03 ± 0.35 nM (n=3), similar to previously reported values [18].
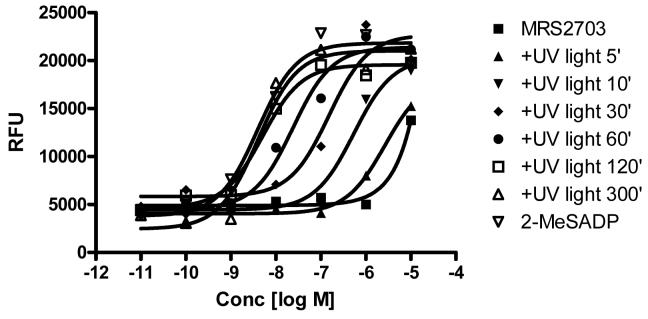
For irradiation of the caged agonist, a dilute solution of MRS2703 (20 μM) in PBS in a quartz cuvette was exposed to 360 nm UV light (from a portable light source equipped with a filter) for a range of times. The production of 2-MeSADP upon irradiation was determined using a bioassay consisting of stimulation of calcium mobilization in astrocytoma cells stably expressing the human P2Y1 receptor. At 10 μM MRS2703, a partial (∼50% of maximal) stimulation of calcium mobilization was noted. The potency of the solution gradually increased over a period of 5 min, such that the final activity reached the same potency and maximal effect as 2-MeSADP (Fig. 3). This indicated that most of the MRS2703 was effectively un-caged upon irradiation to provide the biologically active P2Y1 receptor agonist, 2-MeSADP. As a control, the activity of 2-MeSADP before and after 5 min irradiation under the same condition was not changed. The EC50 values of the MRS2703 after exposure to UV light for 0, 5, 10, 30, 60, 120 and 300 seconds were demonstrated to be ≥10,000, 3420 ± 586, 633 ± 128, 122 ± 36, 22 ± 7, 3.9 ± 1.4 and 2.8 ± 0.7 nM (n=3), respectively. As a control, the EC50 value of 2-MeSADP after 300 seconds exposure to UV light was shown to be 2.6 ± 0.8 nM, which is similar to that of the non-irradiated. The results suggest that the UV light exposure only induces the uncaging not the breakdown of 2-MeSADP.
Figure 3.
Concentration-response curves for the increase in [Ca2+]i induced by the agonist mixtures, upon UV light irradiation, in hP2Y1-1321N1 cells. Cells were loaded with 50 μl dye with 2.5 mM probenecid in each well and incubated for 45 min at room temperature. The compound plate was prepared with dilutions of various compounds in Hank's Buffer at pH 7.2. Samples were run with a Flexstation I (Molecular Devices), and cell fluorescence (excitation = 485 nm; emission = 525 nm) was monitored following exposure to the agonist. Increases in intracellular calcium are reported as the maximum fluorescence value after exposure minus the basal fluorescence value before exposure. The data shown are from an experiment performed in duplicate representative of 2-4 separate experiments of similar results performed in duplicate.
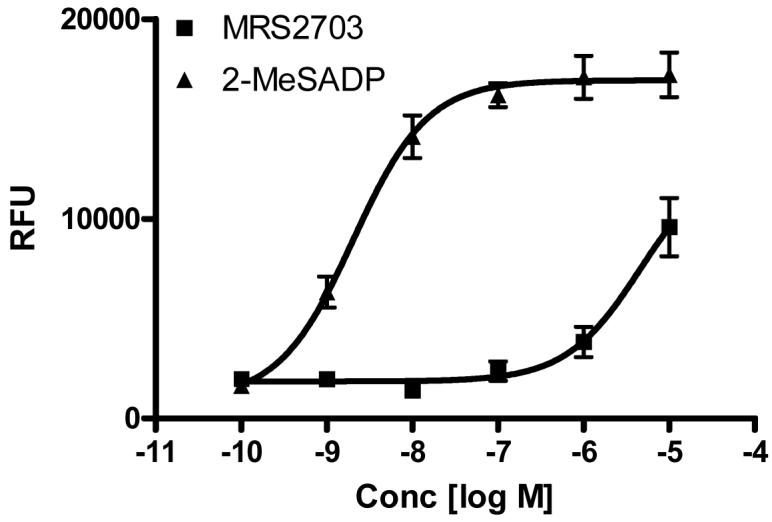
2-MeSADP is an agonist of P2Y1 and P2Y12 receptors. Thus, we next tested if the caged 2-MeSADP, MRS2703, was able to activate P2Y12 receptors, either before or following irradiation. The activity at the P2Y12 receptor was tested using a bioassay consisting of stimulation of calcium mobilization in stably transfected 1321N1 astrocytoma cells. Although in a previous study the 1321N1 cells were cotransfected with a chimeric G protein, Gαq/i, to confer a capacity of the Gi-linked P2Y12 receptor to directly activate phospholipase C [26], in the current study only the endogenous G proteins were present. The coupling of the P2Y12 receptor to a calcium response in the 1321N1 cells was likely as reported previously [17] through G-protein β,γ subunits. Control 1321N1 cells did not display a rise in [Ca2+]i levels in response to 2-MeSADP, but in those cells expressing the P2Y12 receptor 2-MeSADP induced a concentration-dependent rise with an EC50 value of 23 ± 6 nM. Fig. 4 shows that MRS2703, at concentrations up to 10 μM, did not induce a rise in [Ca2+]i levels. However, upon 120 sec irradiation, the un-caged MRS2703 showed an EC50 of 41.4 ± 12.8 nM (n=3).
Figure 4.
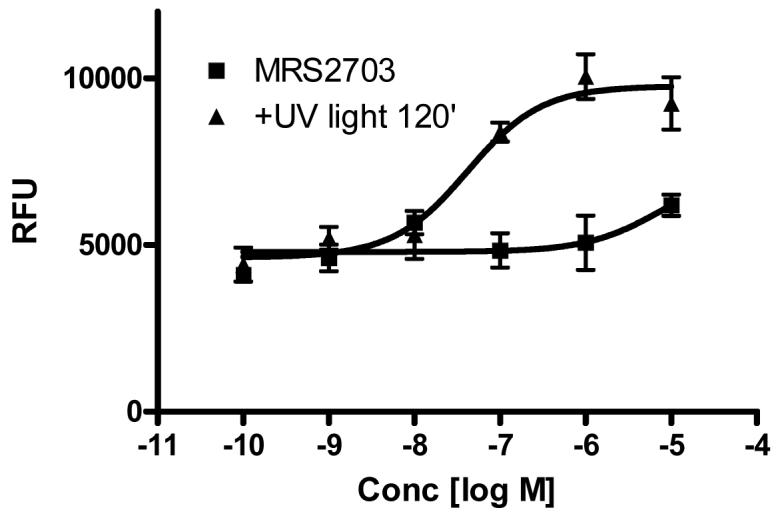
The ability of MRS2703 and the un-caged MRS2703 (upon 120 sec irradiation) to activate the human P2Y12 receptor stably expressed in 1321N1 astrocytoma cells. Cells were loaded with 50 μl dye with 2.5 mM probenecid in each well and incubated for 45 min at room temperature. Activation of the P2Y12 receptor by either 2-MeSADP or the irradiated MRS2703 induced a concentration-dependent increase in [Ca2+]I that was not observed in control 1321N1 astrocytoma cells. The methods used were described in the Materials and Methods. Results are from three separate experiments performed in duplicate.
3.3. Characterization in human platelets
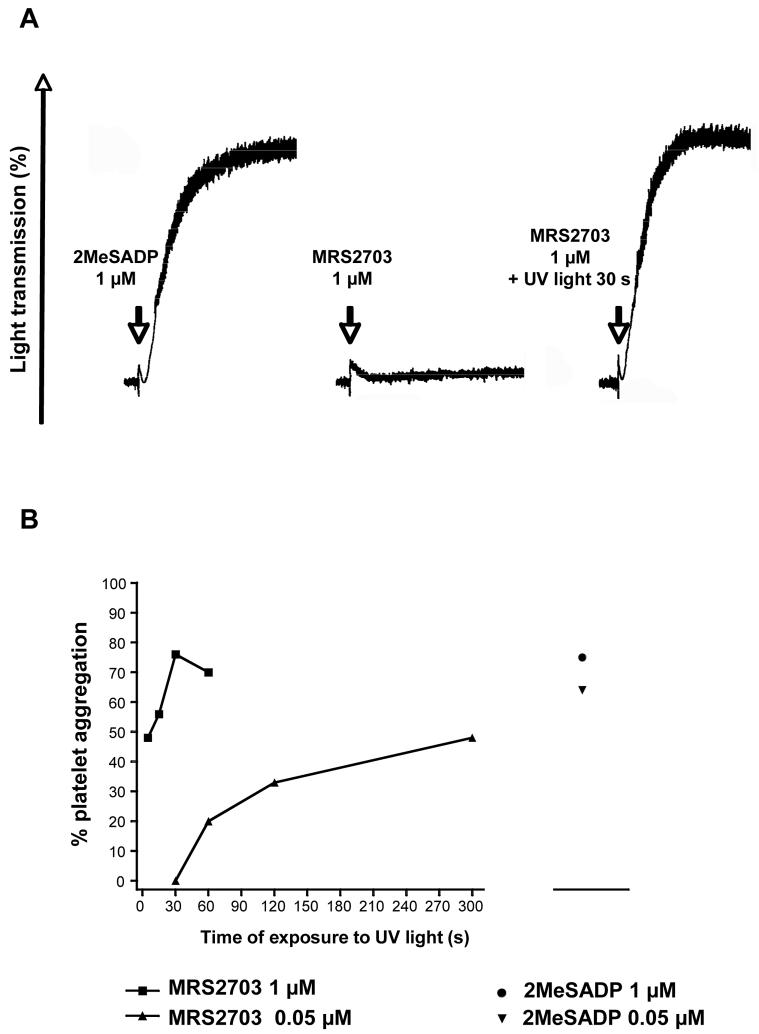
The photoregeneration of 2-MeSADP was then studied in washed human platelets (Fig. 5). MRS2703 (1 μM), without exposure to UV light, did not induce aggregation or shape change of human washed platelets (Fig. 5A). In contrast, exposure of the compound (1 or 0.05 μM) to UV light for 5 s or longer resulted in platelet aggregation (Fig. 5A,B). At the highest concentration of MRS2703 tested (1 μM), full aggregation was achieved within 30 s of irradiation. The extent of aggregation was comparable to aggregation induced by 2-MeSADP at a concentration of 1 μM. The ability to induce platelet aggregation was slower at a lower concentration of MRS2703 (0.05 μM) (Fig. 5B).
Figure 5.
Aggregation of human washed platelets induced by MRS2703 following UV irradiation. A. Representative washed human platelet aggregation measurements. B. Effect of time exposure of MRS2703 (0.05 or 1 μM) to UV light on the extent of platelet aggregation. The methods used were described in the Materials and Methods.
4. Discussion
In the present study, we have synthesized and pharmacologically characterized the nucleotide derivative MRS2703. The photosensitive 1-(3,4-dimethyloxyphenyl)eth-1-yl protecting group was placed at the β-phosphate of 2-MeSADP. This 2-nitrobenzyl-type group selected for “caging” the nucleotide agonist derivative is additionally substituted with 4,5-dimethoxy groups and an α-methyl moiety for light absorption at longer wavelengths and less reactive photo-side products, respectively [2,6]. Prior to irradiation, MRS2703 is inactive at both P2Y1 and P2Y12 receptors. However, upon UV light exposure, it potently activated both P2Y1 and P2Y12 receptors stably expressed in 1321N1 astrocytoma cells, and induced platelet aggregation in vitro. Thus, it is suggested that the caged agonist for P2Y1 and P2Y12 receptors, should be useful in controlling cell signaling. The inactivity of nonirradiated MRS2703 at P2Y1 and P2Y12 receptors is consistent with the hypothesis that blocking the β-phosphate as a terminal ester precludes high affinity binding to these two receptor subtypes. We have not evaluated the light-directed activation of the P2Y13 receptor by MRS2703. However, such activation is expected because the parent nucleotide and photoproduct, 2-MeSADP, is a potent agonist of the P2Y13 receptor [1,26].
Although caged-ADP and caged-ATP have been available previously, they are not selective agonists for the P2Y1 and P2Y12 receptors. Thus, potentially non-specific effects could result using these nonselective agonists. Compared with the non-caged 2-MeSADP, MRS2703 could be metabolically more stable, because it is predicted that caged 2-MeSADP as a β-phosphate ester would be resistant to certain phosphatases by analogy to dinucleotides [21]. Thus, caged 2-MeSADP may be used as a relatively stable source of 2-MeSADP to be used in mechanistic studies.
The caged molecules, such as MRS2703, have the potential to achieve precise temporal control of signaling in mechanistic studies. Investigation of the mechanisms of action of the biological systems, such as cells, requires special techniques to introduce effector molecules rapidly with known concentration and location. Time-resolved measurements on such systems may be impossible if the diffusion of target molecules to the cells is slow or if complications resulted from degradation of the molecules or desensitization of the biological response. One approach to this problem is to introduce the molecule rapidly and uniformly by pulse photolysis of precursor compounds that are biologically inactive. Thus, MRS2703 should be useful for the mechanistic studies of P2Y1 and P2Y12 receptor-related biological functions, such as platelet aggregation.
In a similar manner other nucleotides that act as selective P2Y receptor ligands could be caged for rapid photoregeneration. For example, regioselective conversion of one of the monophosphate groups of the selective P2Y1 antagonist MRS2500, an (N)-methanocarba 3′5′-bisphosphate derivative, to a phosphodiester would be expected to greatly diminish its biological activity. Thus, use of an o-nitrobenzyl-type phosphodiester would allow that monophosphate moiety to be regenerated upon irradiation. Alternately, the P2Y1 selective agonist MRS2365, an (N)-methanocarba derivative of 2MeSADP [26], could be derivatized in a manner analogous to MRS2703 for the selective light-directed activation of P2Y1 receptors. Furthermore, caged P2Y receptor ligands, such as MRS2703 and caged MRS2500 may have potential for hemodynamic control in the clinic, in a manner that is more spatially selective than with use of anti-thrombotic drugs. The effects of the MRS2703 must now be evaluated in in vivo models. Perhaps this probe will be found useful in electrophysiological studies of the brain in which selected regions can be irradiated, by analogy to caged excitatory amino acid derivatives [24]. Methods for the rapid and noninvasive liberation of transmitters using irradiation have been shown to allow the manipulation of biochemical signals in the brain with submicron spatial resolution [24]. The effects of P2Y1 receptors, P2Y12 receptors, and other P2 receptors in cerebral ischemia and in the central nervous system in general are being studied [25]. Using a more sophisticated laser irradiation device, we would expect the in situ photocleavage of MRS2703 to occur on a much faster time scale than noted in this study.
In summary, the newly synthesized nucleotide derivative MRS2703 acts as a caged agonist for both P2Y1 and P2Y12 receptors, which can be rapidly liberated in biologically active form upon brief irradiation with UV light. Thus, MRS2703 and similar derivatives of other P2Y receptor ligands should be useful for precise control of signaling temporally and spatially in future mechanistic studies.
Acknowledgments
This work was supported in part by the Intramural Research Program of NIDDK, National Institutes of Health, Bethesda, MD, USA. We thank Dr. Hyojin Ko (NIH) for HPLC analysis.
Abbreviations
- FBS
fetal bovine serum
- MRS2500
(1′R,2′S,4′S,5′S)-4-(2-iodo-6-methylamino-purin-9-yl)-1-[(phosphato)-methyl]-2-(phosphato)-bicyclo[3.1.0]hexane
- MRS2703
P1-2-(methylthio)adenosyl-P2-(RS)-1-(4,5-dimethoxy-2-nitrophenyl)ethyl pyrophosphate, triethylammonium salt
- 2-MeSADP
2-methylthioadenosine 5′-diphosphate
- DMEM
Dulbecco's modified Eagle's medium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patchornik A, Amit B, Woodward RB. Photosensitive protecting groups. J Am Chem Soc. 1970;92:6333–6335. [Google Scholar]
- 2.Meldrum RA, Shall S, Trentham DR, Wharton CW. Kinetics and mechanism of DNA repair. Preparation, purification and some properties of caged dideoxynucleosides triphosphates. Biochem J. 1990;266:885–890. [PMC free article] [PubMed] [Google Scholar]
- 3.Fodor SP, Read JL, Pirrung MC, Stryer L, Lu AT, Solas D. Light-directed, spatially addressable parallel chemical synthesis. Science. 1991;251:767–73. doi: 10.1126/science.1990438. [DOI] [PubMed] [Google Scholar]
- 4.Kantevari S, Hoang CJ, Ogrodnik J, Egger M, Niggli E, Ellis-Davies GC. Synthesis and two-photon photolysis of 6-(ortho-nitroveratryl)-caged IP3 in living cells. Chembiochem. 2006;7:174–180. doi: 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- 5.Mayer G, Heckel A. Biologically active molecules with a “light switch”. Angew Chem Int Ed Engl. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 6.Golan R, Zehavi U, Naim M, Patchornik A, Smirnoff P, Herchman M. Photoreversible modulators of Escherichia coli beta-galactosidase. 1-Benzoyl-1-cyano-2-(4,5-dimethoxy-2-nitrophenyl)-ethene and 1,1-dicyano-2-(4,5-dimethoxy-2-nitrophenyl)-ethene. J Protein Chem. 2000;19:123–128. doi: 10.1023/a:1007082516503. [DOI] [PubMed] [Google Scholar]
- 7.Patchornik A, Jacobson KA, Strub MP. Photo-reversible affinity labeling. In: van Binst G, editor. Design and Synthesis of Organic Molecules Based on Molecular Recognition, Proceedings of the XVIIIth Solvay Conference on Chemistry. Brussels, Springer; 1986. pp. 235–241. [Google Scholar]
- 9.McCray JA, Herbette L, Kihara T, Trentham DR. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci USA. 1980;77:7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heckel A, Mayer G. Light regulation of aptamer activity: an anti-thrombin aptamer with caged thymidine nucleobases. J Am Chem Soc. 2005;127:822–823. doi: 10.1021/ja043285e. [DOI] [PubMed] [Google Scholar]
- 11.Geissler D, Kresse W, Wiesner B, Bendig J, Kettenmann H, Hagen V. DMACM-caged adenosine nucleotides: ultrafast phototriggers for ATP, ADP, and AMP activated by long-wavelength irradiation. Chembiochem. 2003;4:162–170. doi: 10.1002/cbic.200390027. [DOI] [PubMed] [Google Scholar]
- 12.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, King BF, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII. Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 14.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hechler B, Nonne C, Roh EJ, Cattaneo M, Cazenave JP, Lanza F, Jacobson KA, Gachet C. MRS2500 [2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate], a potent, selective and stable antagonist of the P2Y1 receptor, with strong antithrombotic activity in mice. J Pharm Exp Therap. 2006;316:556–563. doi: 10.1124/jpet.105.094037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maillard MC, Arlinghaus L, Glashofer M, Lee KS, Jacobson KA. Photolabile A1-adenosine receptor agonists as caged electrophysiological probes. Med Chem Res. 1991;1:322–329. [PMC free article] [PubMed] [Google Scholar]
- 17.Mamedova LK, Gao ZG, Jacobson KA. Regulation of death and survival in astrocytes by ADP activating P2Y1 and P2Y12 receptors. Biochem Pharmacol. 2006;72:1031–1041. doi: 10.1016/j.bcp.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niebauer RT, Gao ZG, Li B, Wess J, Jacobson KA. Signaling of the human P2Y1 receptor measured by a yeast growth assay with comparisons to assays of phospholipase C and calcium mobilization in 1321N1 human astrocytoma cells. Purinergic Signal. 2005;1:241–247. doi: 10.1007/s11302-005-6310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan JH, Forbush B, 3rd, Hoffman JF. Rapid photolytic release of adenosine 5′-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978;17:1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- 21.Pojoga LH, Haghiac ML, Moose JE, Hilderman RH. Determination of ATP impurity in adenine dinucleotides. Nucleosides, Nucleotides & Nucleic Acids. 2004;23:581–598. doi: 10.1081/NCN-120030716. [DOI] [PubMed] [Google Scholar]
- 22.Cazenave JP, Ohlmann P, Cassel D, Eckly A, Hechler B, Gachet C. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol Biol. 2004;272:13–28. doi: 10.1385/1-59259-782-3:013. [DOI] [PubMed] [Google Scholar]
- 23.Bourdon DM, Boyer JL, Mahanty S, Jacobson KA, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–868. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoham S, O'Connor DH, Sarkisov DV, Wang SS. Rapid neurotransmitter uncaging in spatially defined patterns. Nat Methods. 2005;2:837–843. doi: 10.1038/nmeth793. [DOI] [PubMed] [Google Scholar]
- 25.Gerevich Z, Zadori Z, Muller C, Wirkner K, Schroder W, Rubini P, Illes P. Metabotropic P2Y receptors inhibit P2X3 receptor-channels via G protein-dependent facilitation of their desensitization. Br J Pharmacol. 2007;151:226–236. doi: 10.1038/sj.bjp.0707217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–1043. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]