Abstract
Various biomarkers have been suggested as associative or predictive of HIV-associated neurocognitive impairment. Plasma levels of monocyte chemoattractant protein 1 (MCP-1), tumor necrosis factor α(TNF-α), and hematocrit were evaluated for relationships with diffusion tensor imaging measurements of centrum semiovale, caudate, and putamen. MCP-1 levels correlated with tissue status (mean diffusivity) in all examined regions. Plasma markers were also significantly correlated with anisotropy measurements in centrum semiovale (TNF-α) and putamen (hematocrit).
Diffusion tension imaging (DTI) exploits the random translational movements of water molecules as a noninvasive mechanism for probing brain regions of interest (ROIs).1 This strategy can be used to derive putative measurements of tissue injury in vivo. Diffusion abnormalities have been detected in patients with HIV, and DTI measurements correlate with cognitive status.2 Markers of immune activation, monocyte chemoattractant protein 1 (MCP-1), and tumor necrosis factor α (TNF-α) and of anemia (hematocrit) have been identified as potential determinants of HIV-dementia (HIV-D).3 This investigation examined relationships between MCP-1, TNF-α, and hematocrit levels in plasma and measurements of the direction-dependent (anisotropy) and mean diffusivity quantified for regions that are vulnerable to injury in patients with HIV, including centrum semiovale, caudate, and putamen.
Methods
Eleven medically stable participants of the Northeast AIDS Dementia cohort study were evaluated (age 49.5 ± 7.3 years; nine men, two women). Exclusion criteria included history of neurologic disorders, stroke, head trauma, opportunistic CNS infection, psychosis at entry, or magnetic resonance contraindications. Seropositivity was confirmed by ELISA and Western blot. CD4 counts ranged from 24 to 427/mm3; plasma viral load ranged from undetectable to 154,938 copies/mL. All subjects were on antiretroviral regimens; nine were receiving protease inhibitors. Memorial Sloan-Kettering (MSK) dementia severity ratings for the sample were as follows: 0.5 (n = 6), 1 (n = 4), and 2 (n = 1). MCP-1 (ng/mL) and TNF-α (pg/mL) levels were determined using commercial kits (Quantikine ELISA).
Magnetic resonance studies were performed on a 1.5-T twin-speed unit (Milwaukee, WI). A quadrature birdcage head coil was used for radio frequency transmission and signal reception. DTI was performed with an echo planar sequence and bandwidth of ±125 kHz using dual spin echo to minimize distortion. Diffusion encoding was applied along six directions with a b-value of 1,000 seconds/mm2. A b = 0 reference image was also acquired. The entire brain was imaged using 22 contiguous 7-mm axial sections (fov 24 cm, matrix 128 × 128, TR/NEX 7,000/4). Custom software was used for image analysis (DPTools, Paris, France). Mean diffusivity and fractional anisotropy were calculated according to standard equations.1 ROI placement is shown in figure 1.
Figure 1.

Regions of interest (ROIs) for putamen and caudate nuclei as shown on an axial slice through interventricular foramen (top). ROIs for centrum semiovale were placed on an axial slice above the bilateral ventricles (bottom). ROI (43 mm2) measurements were acquired in each hemisphere and then averaged.
Results
Relationships between the plasma and DTI variables were evaluated using Pearson correlation coefficients (SPSS, Chicago, IL). For centrum semiovale, correlations were identified between mean diffusivity and MCP-1 (r = −0.69, p = 0.03) and between fractional anisotropy and TNF-α (r =−0.71, p =0.03). For caudate, mean diffusivity was correlated with MCP-1 (r = −0.79, p = 0.007). For putamen, correlations were identified between mean diffusivity and MCP-1 (r = −0.63, p = 0.05) and between fractional anisotropy and hematocrit (r = 0.59, p = 0.05). No other significant relationships were identified.
Discussion
Plasma levels of the β chemokine, MCP-1, were significantly correlated with tissue injury in all brain regions examined, including centrum semiovale, caudate, and putamen. MCP-1 levels in the HIV patients were consistent with the considerably higher than normative values reported in HIV-D.3 MCP-1 measurements were inversely correlated with mean diffusivity in subcortical regions; higher MCP-1 levels generally corresponded to lower mean diffusivity (figure 2).
Figure 2.

Relationship between monocyte chemoattractant protein 1 (MCP-1) and mean diffusivity (MD) for each region.
The mean diffusivity is a biophysical measurement of the apparent mobility of protons in an interrogated region. Membranes, membrane permeability, and the relative volume and morphology of the extracellular space are determinants of this measurement.1 MCP-1 plays a role in both acute and chronic inflammation. Increased plasma MCP-1 levels may correspond to more active or acute inflammation in subcortical regions. Generally, acute inflammatory processes restrict displacements of protons, reducing the mean diffusivity. Glial cell swelling, astrocytosis, hypertrophy of astrocytes and altered extracellular volume owing to distribution of viral proteins and the ionic composition of surrounding cells are possible neuropathologic changes in HIV-D patients4 that may reduce diffusion. Spectroscopy studies have found that markers of glial activation correlate with CSF MCP-1 levels in patients with HIV.5,6
As plasma MCP-1 levels declined, the corresponding mean diffusivity values tended to increase. This pattern may reflect advancing or irreversible injury corresponding to chronic immune activation or burnt-out inflammation. Altered expression of chemokines such as MCP-1 may contribute to neurodegeneration in HIV patients through bystander effects, presumably by attracting monocyte macrophages into the brain, which then release proinflammatory cytokines or neurotoxic HIV proteins.4 Atrophic tissue changes (e.g., apoptosis of astrocytes and neurons in patients with HIV) are associated with expanded extracellular space and increased mobility of protons.1
Significant correlations were also found between plasma markers and anisotropy measurements. Anisotropy measurements reflect injury to highly aligned cellular structures and may be sensitive to white matter alterations (e.g., injury to axons or replacement of axonal fibers with less ordered cells, such as glial cells).1 Reduced hematocrit, a marker of anemia, was significantly correlated with anisotropy measurements acquired in putamen. Anemia is among the limited number of risk factors that have been identified for HIV-D (see McArthur et al.4 for a discussion), and reduced hematocrit has been associated with incident HIV-D in the highly active antiretroviral therapy treatment era.3 These findings implicate white matter alterations in basal ganglia, particularly in putamen, in this risk relationship. Increased TNF-αin plasma was significantly correlated with loss of white matter integrity in centrum semiovale (figure 3). Plasma levels of TNF-α are also predictive of temporal progression to HIV-D.3
Figure 3.
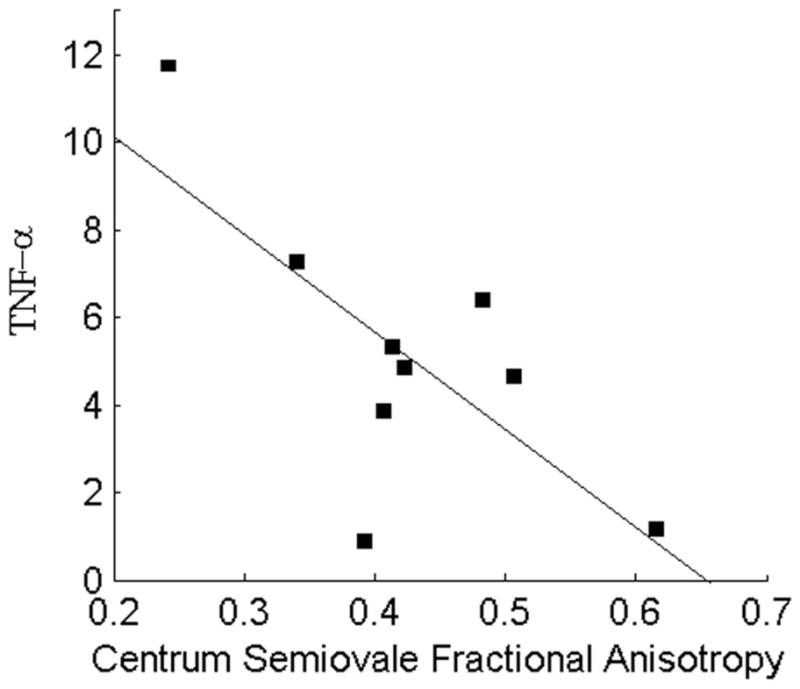
Relationship between tumor necrosis factor α (TNF-α) and anisotropy in deep white matter.
Monocyte chemoattractant protein 1 levels are increased in the CSF of HIV-D patients.7,8 Significant prognostic relationships have been identified between CSF MCP-1 levels and HIV-D; however, findings for plasma MCP-1 have been inconsistent.3,5 MCP-1 levels in plasma may not bear a linear relationship to neurologic progression. It has been suggested, for example, that increased systemic MCP-1 may confer partial protection from initial viral infection but play a detrimental role after infection is established, contributing to accelerated disease progression and increased risk of HIV-D.9 The risk associated with peripheral MCP-1 levels may depend on degree of immunosuppression or other factors. An MCP-1 allele has been identified that is associated with increased levels in serum and a 4.5-fold increased risk of HIV-D.9 This genotype may represent a host factor underlying individual differences in MCP-1 expression and in vulnerability to dementia in patients with HIV.9
Enhanced levels of MCP-1 and of TNF-αin plasma may contribute to brain injury by influencing monocyte ingress in late stages of infection. Escalation in trafficking of activated and HIV-infected monocytes into the brain may initiate prolonged or self-sustaining states of deleterious immune activation.10 Infiltrating monocytes accumulate within the perivascular space, activating microglia and other macrophages. Activated cells produce proinflammatory cytokines and other mediators of inflammation, including chemokines that attract more monocytes into the brain. Activation of brain macrophages and microglia infected with HIV may also initiate virus replication with consequent production of neurotoxic HIV proteins. Prolonged or unrelenting stimulation of macrophages may result in extensive injury to brain tissue. This study identified significant correlations between MCP-1 in plasma and tissue alterations in all studied regions of deep white matter and basal ganglia. Inhibitors of MCP-1 or its principal receptor may represent targets for therapeutic intervention. These findings, however, are based on a small sample of patients, and a larger, longitudinal study will be necessary to clarify prognostic relationships between plasma markers and localized brain injury in patients with HIV.
Acknowledgments
The authors thank Katherine Conant, Justin McArthur, Linda Reisberg, Renee Ochs, and Rachel Scheidegger and the NEAD consortium.
The National Institute of Mental Health (MH66705) and the National Institute of Neurological Disorders and Stroke (NS36519 and NS049465) provided funding or support for this study.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 2.Ragin AB, Storey P, Cohen BA, Edelman RR, Epstein LG. Disease burden in HIV-associated cognitive impairment: a study of whole brain imaging measures. Neurology. 2004;63:2293–2297. doi: 10.1212/01.wnl.0000147477.44791.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated Dementia. Neurology. 2004;63:2084–2090. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 4.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 5.Chang L, Lee PL, Yiannoutsos CT, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23:1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 6.Avison MJ, Nath A, Greene-Avison R, et al. Inflammatory changes and breakdown of microvascular integrity in early human immunodeficiency virus dementia. J Neurovirol. 2004;10:223–232. doi: 10.1080/13550280490463532. [DOI] [PubMed] [Google Scholar]
- 7.Conant K, Garzino-Demo A, Nath A, et al. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez E, Rovin BH, Sen L, et al. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci USA. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner S. HIV infection and dementia. Science. 2000;287:602–604. doi: 10.1126/science.287.5453.602. [DOI] [PubMed] [Google Scholar]


