Abstract
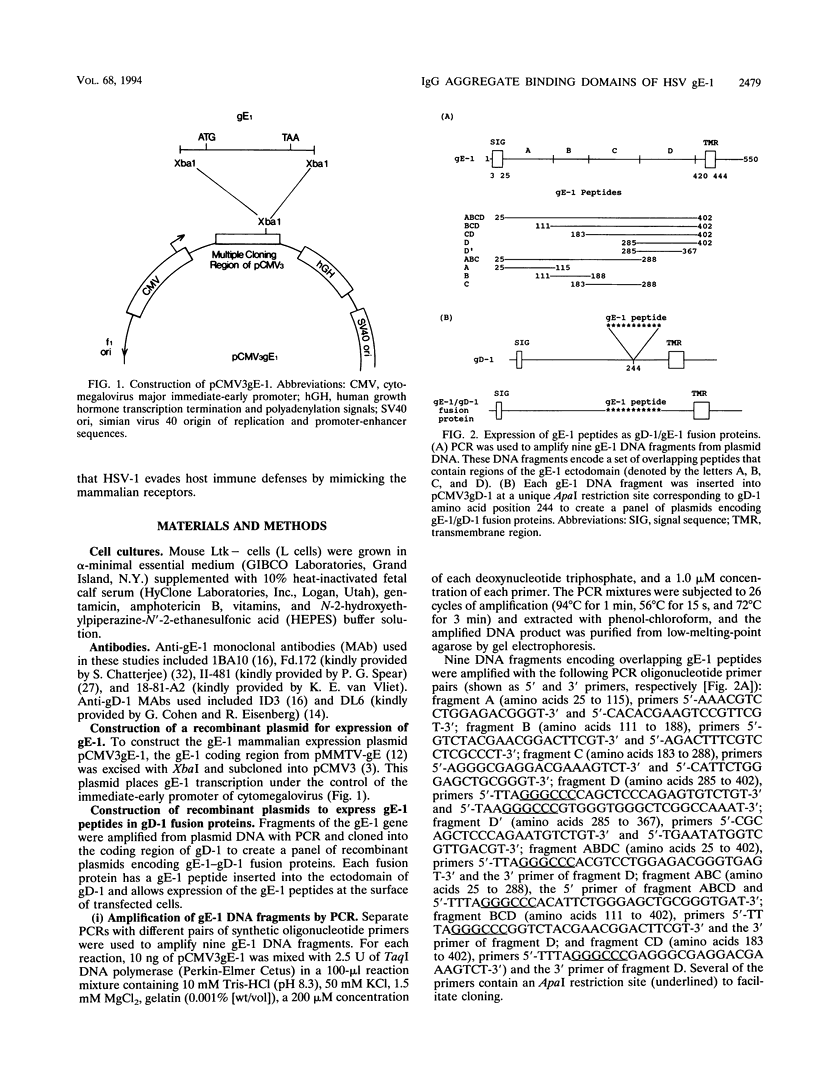
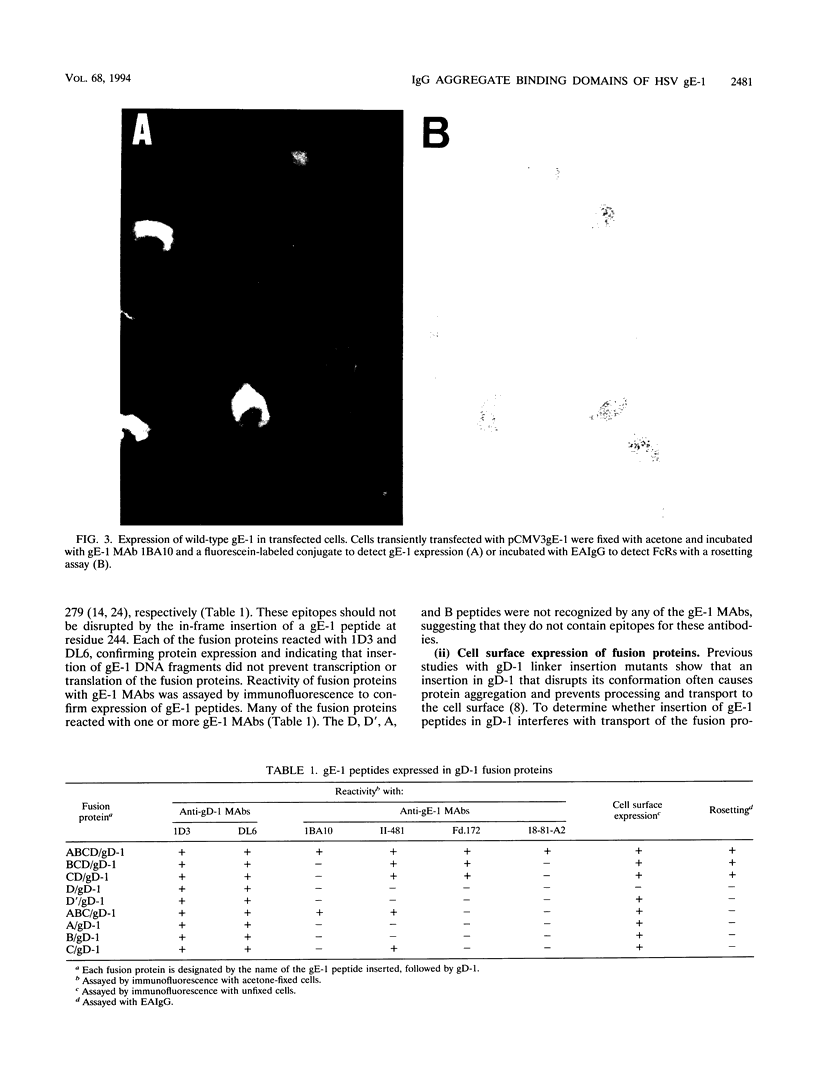
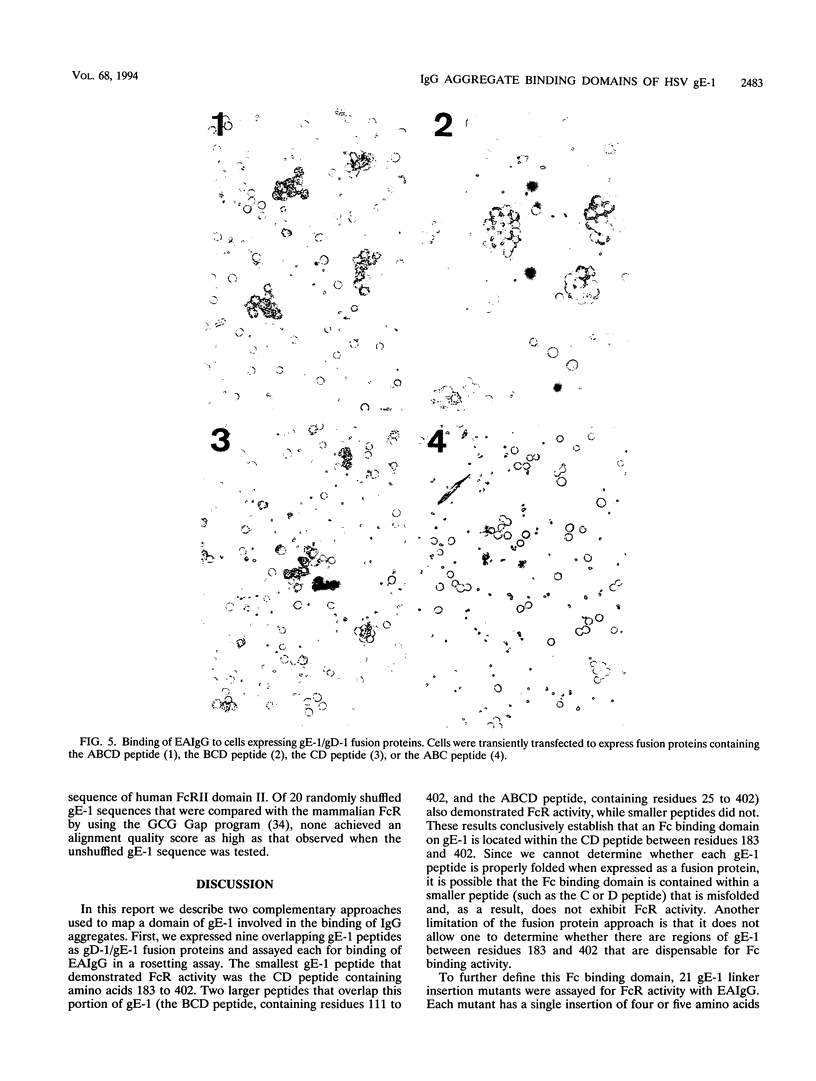
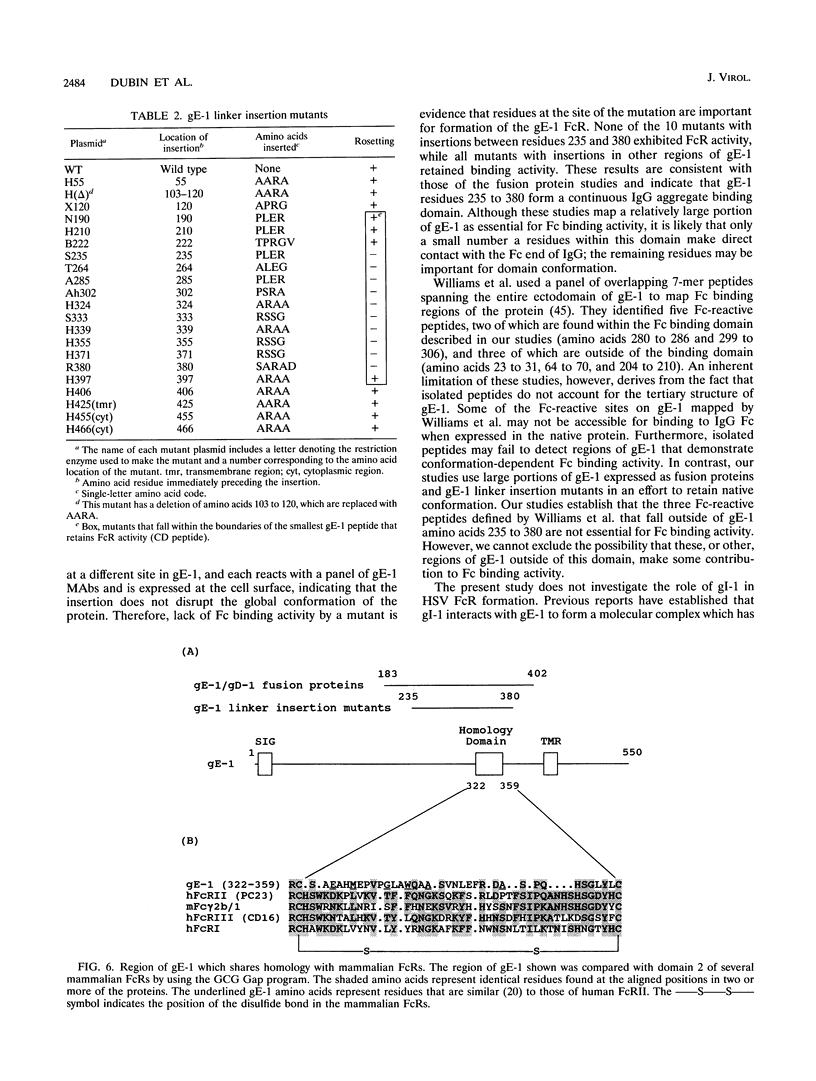
Herpes simplex virus type 1 glycoproteins gE and gI form receptors for the Fc domain of immunoglobulin G (IgG) which are expressed on the surface of infected cells and on the virion envelope and which protect the virus from immune attack. Glycoprotein gE-1 is a low-affinity Fc receptor (FcR) that binds IgG aggregates, while gE-1 and gI-1 form a complex which serves as a higher-affinity FcR capable of binding IgG monomers. In this study, we describe two approaches used to map an Fc binding domain on gE-1 for IgG aggregates. First, we constructed nine plasmids encoding gE-1/gD-1 fusions proteins, each containing a large gE-1 peptide inserted into the ectodomain of gD-1. Fusion proteins were tested for FcR activity with IgG-sensitized erythrocytes in a rosetting assay. Three of the fusion proteins containing overlapping gE-1 peptides demonstrated FcR activity; the smallest peptide that retained Fc binding activity includes gE-1 amino acids 183 to 402. These results indicate that an Fc binding domain is located between gE-1 amino acids 183 and 402. To more precisely map the Fc binding domain, we tested a panel of 21 gE-1 linker insertion mutants. Ten mutants with insertions between gE-1 amino acids 235 and 380 failed to bind IgG-sensitized erythrocytes, while each of the remaining mutants demonstrated wild-type Fc binding activity. Taken together, these results indicate that the region of gE-1 between amino acids 235 and 380 forms an FcR domain. A computer-assisted analysis of the amino acid sequence of gE-1 demonstrates an immunoglobulin-like domain contained within this region (residues 322 to 359) which shares homology with mammalian FcRs.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., Glorioso J. C., Cossman J., Levine M. Possible role of Fc receptors on cells infected and transformed by herpesvirus: escape from immune cytolysis. Infect Immun. 1978 Aug;21(2):442–447. doi: 10.1128/iai.21.2.442-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. M., Seed B. Isolation and expression of functional high-affinity Fc receptor complementary DNAs. Science. 1989 Jan 20;243(4889):378–381. doi: 10.1126/science.2911749. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S., Cranage M., Borysiewicz L., Minson T. Induction of immunoglobulin G Fc receptors by recombinant vaccinia viruses expressing glycoproteins E and I of herpes simplex virus type 1. J Virol. 1990 May;64(5):2181–2186. doi: 10.1128/jvi.64.5.2181-2186.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowler K. W., Veltri R. W. In vitro neutralization of HSV-2: inhibition by binding of normal IgG and purified Fc to virion Fc receptor (FcR). J Med Virol. 1984;13(3):251–259. doi: 10.1002/jmv.1890130307. [DOI] [PubMed] [Google Scholar]
- Dubin G., Frank I., Friedman H. M. Herpes simplex virus type 1 encodes two Fc receptors which have different binding characteristics for monomeric immunoglobulin G (IgG) and IgG complexes. J Virol. 1990 Jun;64(6):2725–2731. doi: 10.1128/jvi.64.6.2725-2731.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin G., Socolof E., Frank I., Friedman H. M. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J Virol. 1991 Dec;65(12):7046–7050. doi: 10.1128/jvi.65.12.7046-7050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Long D., Ponce de Leon M., Matthews J. T., Spear P. G., Gibson M. G., Lasky L. A., Berman P., Golub E., Cohen G. H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985 Feb;53(2):634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank I., Friedman H. M. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J Virol. 1989 Nov;63(11):4479–4488. doi: 10.1128/jvi.63.11.4479-4488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H. M., Cohen G. H., Eisenberg R. J., Seidel C. A., Cines D. B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature. 1984 Jun 14;309(5969):633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hanke T., Graham F. L., Lulitanond V., Johnson D. C. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology. 1990 Aug;177(2):437–444. doi: 10.1016/0042-6822(90)90507-n. [DOI] [PubMed] [Google Scholar]
- Harris S. L., Frank I., Yee A., Cohen G. H., Eisenberg R. J., Friedman H. M. Glycoprotein C of herpes simplex virus type 1 prevents complement-mediated cell lysis and virus neutralization. J Infect Dis. 1990 Aug;162(2):331–337. doi: 10.1093/infdis/162.2.331. [DOI] [PubMed] [Google Scholar]
- Hoekstra M. F., Liskay R. M., Ou A. C., DeMaggio A. J., Burbee D. G., Heffron F. HRR25, a putative protein kinase from budding yeast: association with repair of damaged DNA. Science. 1991 Aug 30;253(5023):1031–1034. doi: 10.1126/science.1887218. [DOI] [PubMed] [Google Scholar]
- Hsu D. H., de Waal Malefyt R., Fiorentino D. F., Dang M. N., Vieira P., de Vries J., Spits H., Mosmann T. R., Moore K. W. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990 Nov 9;250(4982):830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- Huemer H. P., Larcher C., Coe N. E. Pseudorabies virus glycoprotein III derived from virions and infected cells binds to the third component of complement. Virus Res. 1992 May;23(3):271–280. doi: 10.1016/0168-1702(92)90113-n. [DOI] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Isola V. J., Eisenberg R. J., Siebert G. R., Heilman C. J., Wilcox W. C., Cohen G. H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989 May;63(5):2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V., Grose C. Herpesviral Fc receptors and their relationship to the human Fc receptors. Immunol Res. 1992;11(3-4):226–238. doi: 10.1007/BF02919129. [DOI] [PubMed] [Google Scholar]
- Litwin V., Jackson W., Grose C. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J Virol. 1992 Jun;66(6):3643–3651. doi: 10.1128/jvi.66.6.3643-3651.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin V., Sandor M., Grose C. Cell surface expression of the varicella-zoster virus glycoproteins and Fc receptor. Virology. 1990 Sep;178(1):263–272. doi: 10.1016/0042-6822(90)90402-d. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- Metcalf J. F., Chatterjee S., Koga J., Whitley R. J. Protection against herpetic ocular disease by immunotherapy with monoclonal antibodies to herpes simplex virus glycoproteins. Intervirology. 1988;29(1):39–49. doi: 10.1159/000150027. [DOI] [PubMed] [Google Scholar]
- Muggeridge M. I., Wu T. T., Johnson D. C., Glorioso J. C., Eisenberg R. J., Cohen G. H. Antigenic and functional analysis of a neutralization site of HSV-1 glycoprotein D. Virology. 1990 Feb;174(2):375–387. doi: 10.1016/0042-6822(90)90091-5. [DOI] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravetch J. V., Luster A. D., Weinshank R., Kochan J., Pavlovec A., Portnoy D. A., Hulmes J., Pan Y. C., Unkeless J. C. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986 Nov 7;234(4777):718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Eisenberg R. J., Cohen G. H. Identification of C3b-binding regions on herpes simplex virus type 2 glycoprotein C. J Virol. 1990 May;64(5):1897–1906. doi: 10.1128/jvi.64.5.1897-1906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin J. M., Desgranges C., Seigneurin D., Paire J., Renversez J. C., Jacquemont B., Micouin C. Herpes simplex virus glycoprotein D: human monoclonal antibody produced by bone marrow cell line. Science. 1983 Jul 8;221(4606):173–175. doi: 10.1126/science.6304881. [DOI] [PubMed] [Google Scholar]
- Simmons D., Seed B. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature. 1988 Jun 9;333(6173):568–570. doi: 10.1038/333568a0. [DOI] [PubMed] [Google Scholar]
- Stengelin S., Stamenkovic I., Seed B. Isolation of cDNAs for two distinct human Fc receptors by ligand affinity cloning. EMBO J. 1988 Apr;7(4):1053–1059. doi: 10.1002/j.1460-2075.1988.tb02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal-Singer R., Seidel-Dugan C., Fries L., Huemer H. P., Eisenberg R. J., Cohen G. H., Friedman H. M. Herpes simplex virus glycoprotein C is a receptor for complement component iC3b. J Infect Dis. 1991 Oct;164(4):750–753. doi: 10.1093/infdis/164.4.750. [DOI] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J. F. ADSORPTION OF SENSITIZED SHEEP ERYTHROCYTES TO HELA CELLS INFECTED WITH HERPES SIMPLEX VIRUS. Nature. 1964 Jun 27;202:1364–1365. doi: 10.1038/2021364a0. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Weis J. H., Salstrom J. S., Enquist L. W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982 Oct 22;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Kievit E., Tsuchiya N., Malone C., Hutt-Fletcher L. Differential mapping of Fc gamma-binding and monoclonal antibody-reactive epitopes on gE, the Fc gamma-binding glycoprotein of herpes simplex virus type 1. J Immunol. 1992 Oct 1;149(7):2415–2427. [PubMed] [Google Scholar]