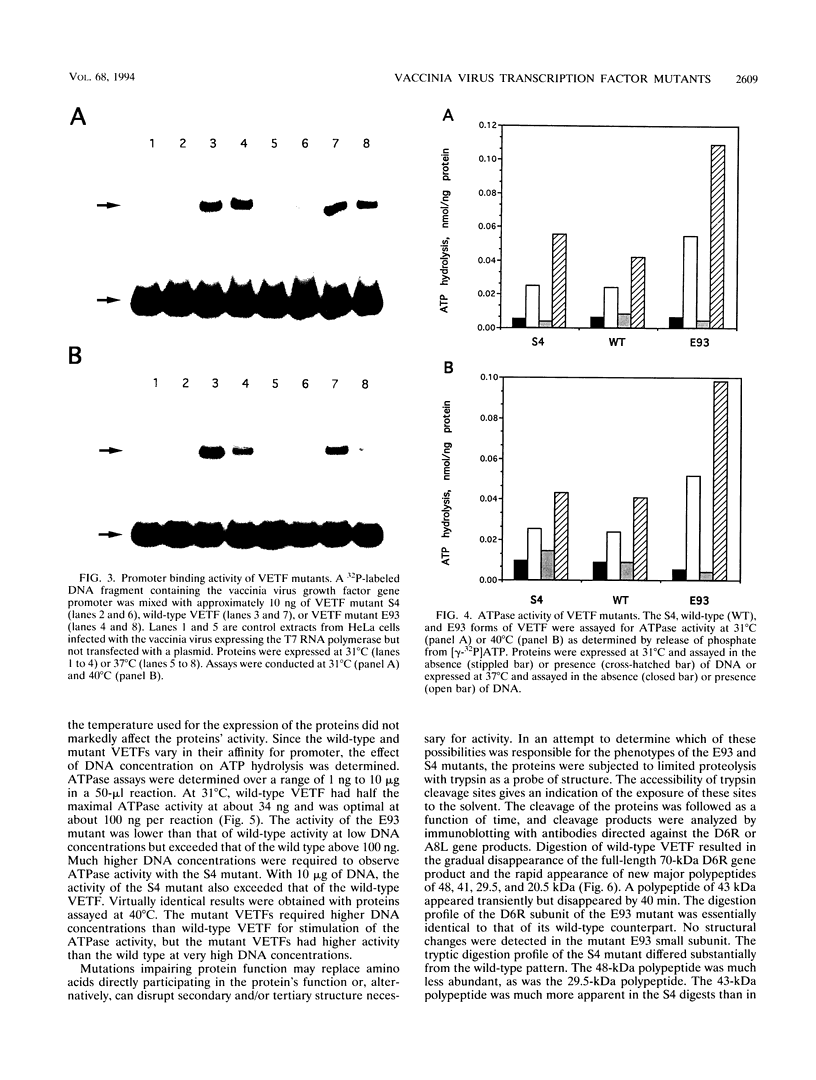
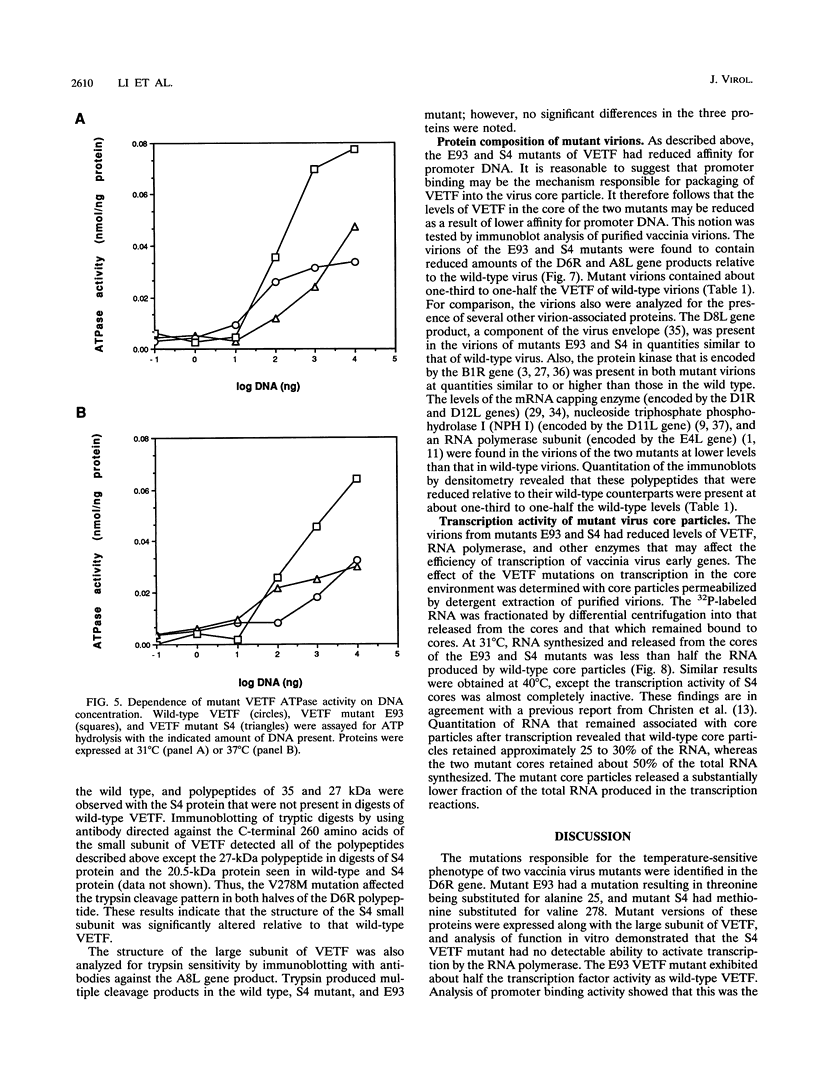
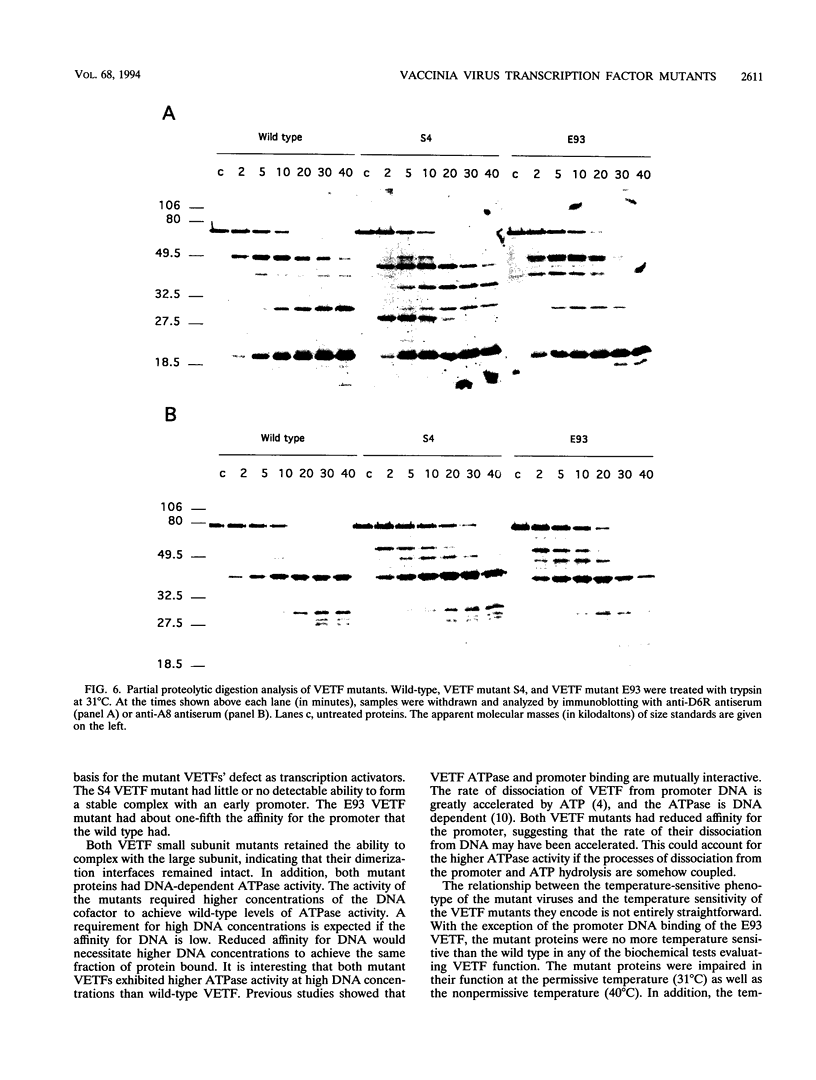
Abstract
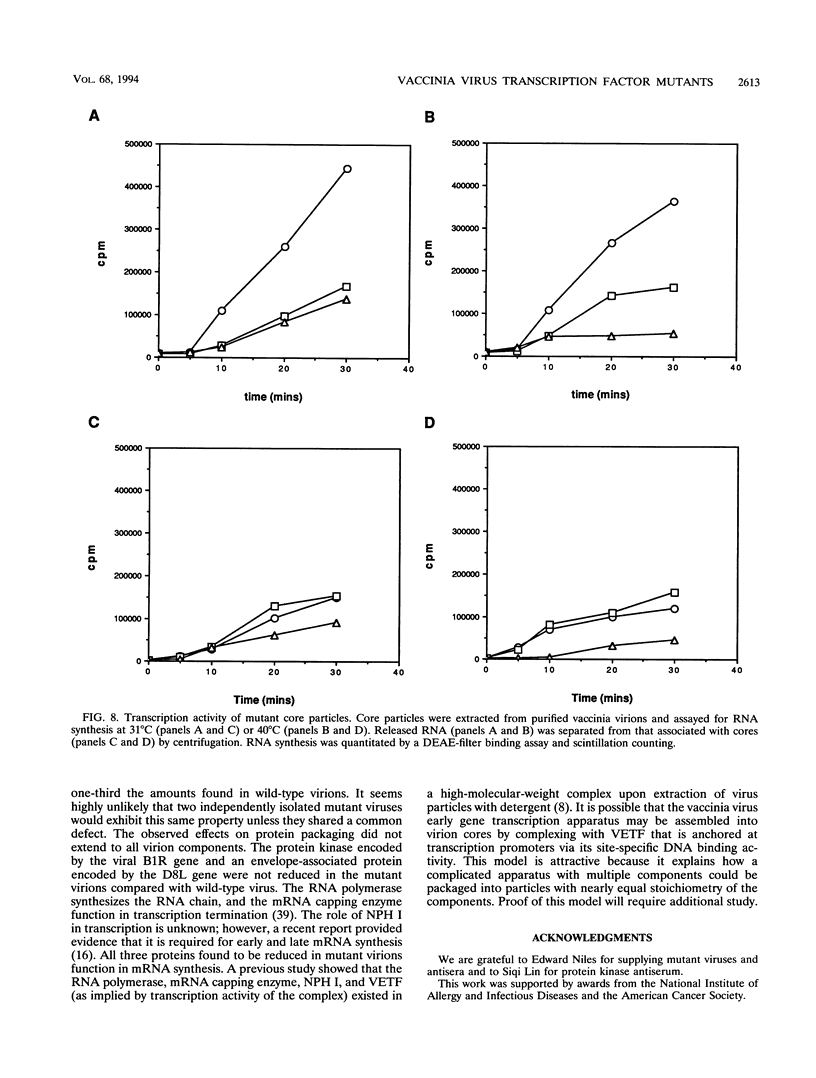
The vaccinia virus D6R open reading frame encodes the small subunit of the heterodimeric vaccinia virus early transcription factor (VETF) that activates transcription of early genes in vitro. VETF binds early gene promoters and has a DNA-dependent ATPase activity that is essential for activation of transcription. To examine the relationship between the structure and function of VETF, we have localized the mutations in two temperature-sensitive viruses whose lesions previously were mapped to the D6R gene. For both mutants, a single G-to-A nucleotide change that would alter protein coding potential was identified. In mutant E93, the codon for alanine 25 was changed to that of threonine, and in mutant S4 the codon for valine 278 was replaced with that for methionine. The molecular phenotype of each mutant was assessed by expressing mutant transcription factors in HeLa cells by using a vaccinia virus-T7 system and characterizing the proteins' activities in vitro. The A25T mutant activated transcription to a lesser extent than wild-type VETF, and the V278M mutant had no demonstrable transcription factor activity. Both mutant proteins were shown to be defective for promoter binding, accounting for their impairment in transcription activation. The functional defects for both mutants were observed at permissive as well as nonpermissive temperatures. The mutant proteins retained ATPase activity but required higher DNA concentrations to activate the ATPase. These results indicate that the small subunit of VETF is essential for its promoter binding activity and likely contacts the promoter DNA. Immunoblotting experiments showed that the virion particles from the two mutant viruses contained about half the VETF of wild-type virus, suggesting that promoter binding may contribute to packaging of VETF into the virion particle. RNA polymerase, mRNA capping enzyme, and nucleoside triphosphate phosphohydrolase I were found at similarly reduced levels in the virion, indicating that packaging of some virion core enzymes may be interdependent.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn B. Y., Gershon P. D., Jones E. V., Moss B. Identification of rpo30, a vaccinia virus RNA polymerase gene with structural similarity to a eucaryotic transcription elongation factor. Mol Cell Biol. 1990 Oct;10(10):5433–5441. doi: 10.1128/mcb.10.10.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn B. Y., Moss B. RNA polymerase-associated transcription specificity factor encoded by vaccinia virus. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3536–3540. doi: 10.1073/pnas.89.8.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banham A. H., Smith G. L. Vaccinia virus gene B1R encodes a 34-kDa serine/threonine protein kinase that localizes in cytoplasmic factories and is packaged into virions. Virology. 1992 Dec;191(2):803–812. doi: 10.1016/0042-6822(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Broyles S. S. A role for ATP hydrolysis in vaccinia virus early gene transcription. Dissociation of the early transcription factor-promoter complex. J Biol Chem. 1991 Aug 15;266(23):15545–15548. [PubMed] [Google Scholar]
- Broyles S. S., Fesler B. S. Vaccinia virus gene encoding a component of the viral early transcription factor. J Virol. 1990 Apr;64(4):1523–1529. doi: 10.1128/jvi.64.4.1523-1529.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Li J., Moss B. Promoter DNA contacts made by the vaccinia virus early transcription factor. J Biol Chem. 1991 Aug 15;266(23):15539–15544. [PubMed] [Google Scholar]
- Broyles S. S., Li J. The small subunit of the vaccinia virus early transcription factor contacts the transcription promoter DNA. J Virol. 1993 Sep;67(9):5677–5680. doi: 10.1128/jvi.67.9.5677-5680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. DNA-dependent ATPase activity associated with vaccinia virus early transcription factor. J Biol Chem. 1988 Aug 5;263(22):10761–10765. [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Identification of the vaccinia virus gene encoding nucleoside triphosphate phosphohydrolase I, a DNA-dependent ATPase. J Virol. 1987 May;61(5):1738–1742. doi: 10.1128/jvi.61.5.1738-1742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Moss B. Sedimentation of an RNA polymerase complex from vaccinia virus that specifically initiates and terminates transcription. Mol Cell Biol. 1987 Jan;7(1):7–14. doi: 10.1128/mcb.7.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Pennington M. J. Vaccinia virus gene encoding a 30-kilodalton subunit of the viral DNA-dependent RNA polymerase. J Virol. 1990 Nov;64(11):5376–5382. doi: 10.1128/jvi.64.11.5376-5382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyles S. S., Yuen L., Shuman S., Moss B. Purification of a factor required for transcription of vaccinia virus early genes. J Biol Chem. 1988 Aug 5;263(22):10754–10760. [PubMed] [Google Scholar]
- Christen L., Higman M. A., Niles E. G. Phenotypic characterization of three temperature-sensitive mutations in the vaccinia virus early gene transcription initiation factor. J Gen Virol. 1992 Dec;73(Pt 12):3155–3167. doi: 10.1099/0022-1317-73-12-3155. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Motyczka A., Spizz G. Isolation, characterization, and physical mapping of temperature-sensitive mutants of vaccinia virus. Virology. 1983 Jul 30;128(2):429–443. doi: 10.1016/0042-6822(83)90268-4. [DOI] [PubMed] [Google Scholar]
- Diaz-Guerra M., Esteban M. Vaccinia virus nucleoside triphosphate phosphohydrolase I controls early and late gene expression by regulating the rate of transcription. J Virol. 1993 Dec;67(12):7561–7572. doi: 10.1128/jvi.67.12.7561-7572.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensinger M. J. Isolation and genetic characterization of temperature-sensitive mutants of vaccinia virus WR. J Virol. 1982 Sep;43(3):778–790. doi: 10.1128/jvi.43.3.778-790.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon P. D., Moss B. Early transcription factor subunits are encoded by vaccinia virus late genes. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4401–4405. doi: 10.1073/pnas.87.11.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler J., Shuman S. Structural analysis of ternary complexes of vaccinia RNA polymerase. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10099–10103. doi: 10.1073/pnas.89.21.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kane E. M., Shuman S. Temperature-sensitive mutations in the vaccinia virus H4 gene encoding a component of the virion RNA polymerase. J Virol. 1992 Oct;66(10):5752–5762. doi: 10.1128/jvi.66.10.5752-5762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsushika S., Kawabe J., Homcy C. J., Ishikawa Y. In vivo generation of an adenylylcyclase isoform with a half-molecule motif. J Biol Chem. 1993 Feb 5;268(4):2273–2276. [PubMed] [Google Scholar]
- Keck J. G., Baldick C. J., Jr, Moss B. Role of DNA replication in vaccinia virus gene expression: a naked template is required for transcription of three late trans-activator genes. Cell. 1990 Jun 1;61(5):801–809. doi: 10.1016/0092-8674(90)90190-p. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Li J., Broyles S. S. The DNA-dependent ATPase activity of vaccinia virus early gene transcription factor is essential for its transcription activation function. J Biol Chem. 1993 Sep 25;268(27):20016–20021. [PubMed] [Google Scholar]
- Lin S., Chen W., Broyles S. S. The vaccinia virus B1R gene product is a serine/threonine protein kinase. J Virol. 1992 May;66(5):2717–2723. doi: 10.1128/jvi.66.5.2717-2723.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. Intramolecular homologous recombination in cells infected with temperature-sensitive mutants of vaccinia virus. J Virol. 1989 May;63(5):2030–2035. doi: 10.1128/jvi.63.5.2030-2035.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. R., Cohen L. K., Roberts B. E. Identification of the DNA sequences encoding the large subunit of the mRNA-capping enzyme of vaccinia virus. J Virol. 1984 Oct;52(1):206–214. doi: 10.1128/jvi.52.1.206-214.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Ahn B. Y., Amegadzie B., Gershon P. D., Keck J. G. Cytoplasmic transcription system encoded by vaccinia virus. J Biol Chem. 1991 Jan 25;266(3):1355–1358. [PubMed] [Google Scholar]
- Moss B. Regulation of vaccinia virus transcription. Annu Rev Biochem. 1990;59:661–688. doi: 10.1146/annurev.bi.59.070190.003305. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Lee-Chen G. J., Shuman S., Moss B., Broyles S. S. Vaccinia virus gene D12L encodes the small subunit of the viral mRNA capping enzyme. Virology. 1989 Oct;172(2):513–522. doi: 10.1016/0042-6822(89)90194-3. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Seto J. Vaccinia virus gene D8 encodes a virion transmembrane protein. J Virol. 1988 Oct;62(10):3772–3778. doi: 10.1128/jvi.62.10.3772-3778.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel R. E., Traktman P. Vaccinia virus B1 kinase: phenotypic analysis of temperature-sensitive mutants and enzymatic characterization of recombinant proteins. J Virol. 1992 Jul;66(7):4413–4426. doi: 10.1128/jvi.66.7.4413-4426.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. F., Kahn J. S., Esteban M. Molecular cloning, encoding sequence, and expression of vaccinia virus nucleic acid-dependent nucleoside triphosphatase gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9566–9570. doi: 10.1073/pnas.83.24.9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J., Celenza L. M., Condit R. C., Niles E. G. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987 Sep;160(1):110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Shuman S., Broyles S. S., Moss B. Purification and characterization of a transcription termination factor from vaccinia virions. J Biol Chem. 1987 Sep 5;262(25):12372–12380. [PubMed] [Google Scholar]
- Shuman S., Moss B. Bromouridine triphosphate inhibits transcription termination and mRNA release by vaccinia virions. J Biol Chem. 1989 Dec 15;264(35):21356–21360. [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]