Abstract
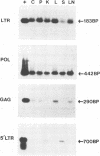
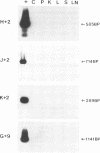
The distribution and replicative status of equine infectious anemia virus (EIAV) DNA in the tissues of a well-characterized inapparent carrier horse were established by using the PCR technique. The EIAV pol region could be amplified in all of the tissues tested, including the cerebellum and periventricular tissue, at concentrations approximately 10(5)-fold less than in the same tissue from an acutely infected horse. Further analysis of the EIAV genome, with primer pairs diagnostic for sequential stages of reverse transcription, suggests that EIAV DNA in the brain, liver, and lymph nodes was incompletely synthesized. The products of reverse transcription were found to diminish progressively during first-strand synthesis, while products indicative of second-strand synthesis were observed only in kidney and spleen DNA samples. Sequences specific for different regions of the envelope could not be amplified from any of the tissues of the inapparent carrier, suggesting that the envelope is highly variable and may be subject to extensive drift. Together, the data suggest that low levels of EIAV replication persist without causing clinical disease in an inapparent carrier.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carpenter S., Chesebro B. Change in host cell tropism associated with in vitro replication of equine infectious anemia virus. J Virol. 1989 Jun;63(6):2492–2496. doi: 10.1128/jvi.63.6.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter S., Evans L. H., Sevoian M., Chesebro B. Role of the host immune response in selection of equine infectious anemia virus variants. J Virol. 1987 Dec;61(12):3783–3789. doi: 10.1128/jvi.61.12.3783-3789.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins L. Carriers of equine infectious anemia virus. J Am Vet Med Assoc. 1984 Feb 1;184(3):279–281. [PubMed] [Google Scholar]
- Coggins L. Mechanism of viral persistence in equine infectious anemia. Cornell Vet. 1975 Apr;65(2):143–151. [PubMed] [Google Scholar]
- Hussain K. A., Issel C. J., Schnorr K. L., Rwambo P. M., Montelaro R. C. Antigenic analysis of equine infectious anemia virus (EIAV) variants by using monoclonal antibodies: epitopes of glycoprotein gp90 of EIAV stimulate neutralizing antibodies. J Virol. 1987 Oct;61(10):2956–2961. doi: 10.1128/jvi.61.10.2956-2961.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Casey J. W. Genomic variation and segregation of equine infectious anemia virus during acute infection. J Virol. 1992 Jun;66(6):3879–3882. doi: 10.1128/jvi.66.6.3879-3882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumi K., Conway B., Cunningham S., Berson A., Evans C., Iversen A. K., Colvin D., Gallo M. V., Coutre S., Shpaer E. G. Human immunodeficiency virus type 1 envelope gene structure and diversity in vivo and after cocultivation in vitro. J Virol. 1992 Feb;66(2):875–885. doi: 10.1128/jvi.66.2.875-885.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Immunofluorescent localization of equine infectious anemia virus in tissue. Am J Pathol. 1971 Feb;62(2):283–294. [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Orrego A., Issel C. J., Montelaro R. C., Adams W. V., Jr Virulence and in vitro growth of a cell-adapted strain of equine infectious anemia virus after serial passage in ponies. Am J Vet Res. 1982 Sep;43(9):1556–1560. [PubMed] [Google Scholar]
- Payne S. L., Fang F. D., Liu C. P., Dhruva B. R., Rwambo P., Issel C. J., Montelaro R. C. Antigenic variation and lentivirus persistence: variations in envelope gene sequences during EIAV infection resemble changes reported for sequential isolates of HIV. Virology. 1987 Dec;161(2):321–331. doi: 10.1016/0042-6822(87)90124-3. [DOI] [PubMed] [Google Scholar]
- Payne S. L., Salinovich O., Nauman S. M., Issel C. J., Montelaro R. C. Course and extent of variation of equine infectious anemia virus during parallel persistent infections. J Virol. 1987 Apr;61(4):1266–1270. doi: 10.1128/jvi.61.4.1266-1270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice N. R., Lequarre A. S., Casey J. W., Lahn S., Stephens R. M., Edwards J. Viral DNA in horses infected with equine infectious anemia virus. J Virol. 1989 Dec;63(12):5194–5200. doi: 10.1128/jvi.63.12.5194-5200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow K., Olsen K., Stiegler G., Payne S. L., Montelaro R. C., Issel C. J. Lentivirus genomic organization: the complete nucleotide sequence of the env gene region of equine infectious anemia virus. Virology. 1986 Dec;155(2):309–321. doi: 10.1016/0042-6822(86)90195-9. [DOI] [PubMed] [Google Scholar]
- Saag M. S., Hahn B. H., Gibbons J., Li Y., Parks E. S., Parks W. P., Shaw G. M. Extensive variation of human immunodeficiency virus type-1 in vivo. Nature. 1988 Aug 4;334(6181):440–444. doi: 10.1038/334440a0. [DOI] [PubMed] [Google Scholar]
- Stephens R. M., Casey J. W., Rice N. R. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science. 1986 Feb 7;231(4738):589–594. doi: 10.1126/science.3003905. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]