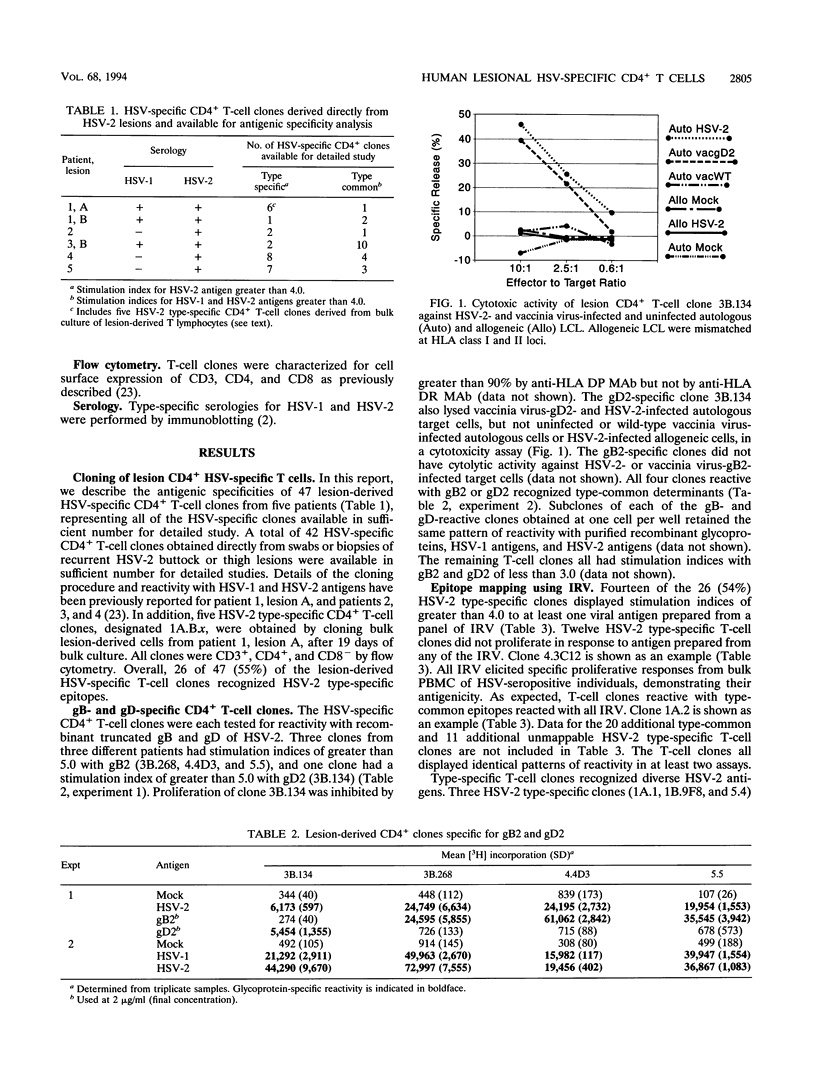
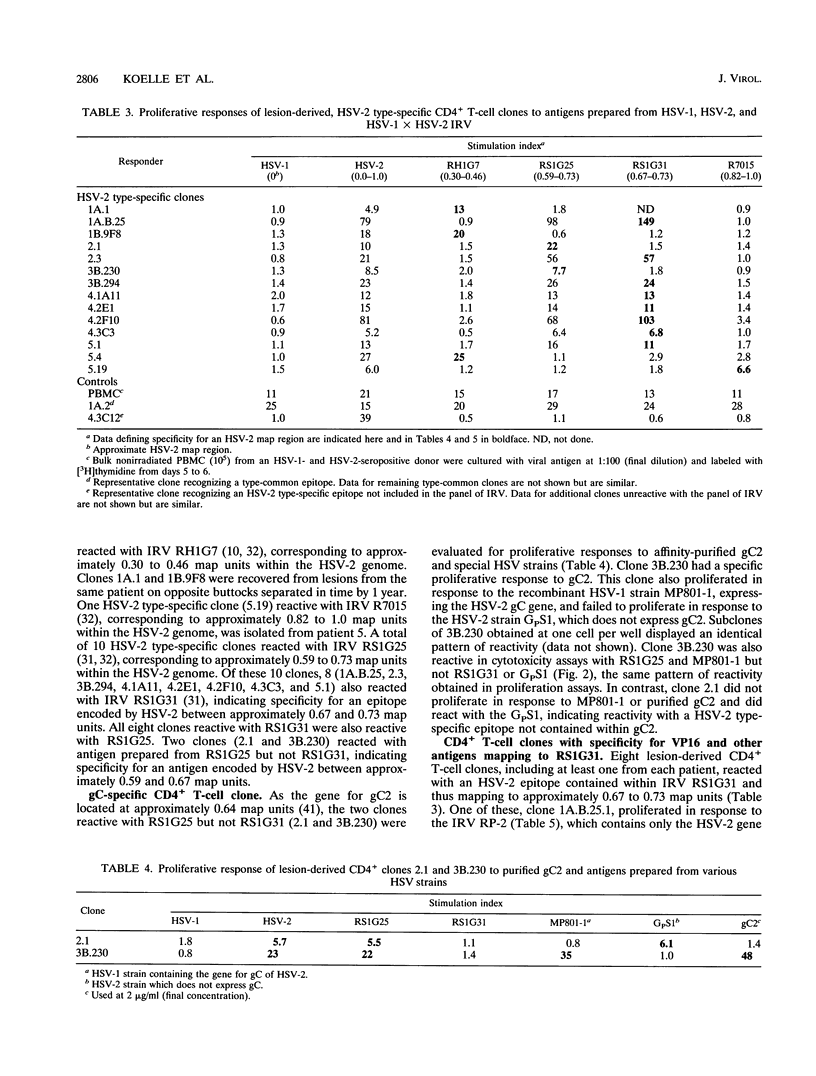
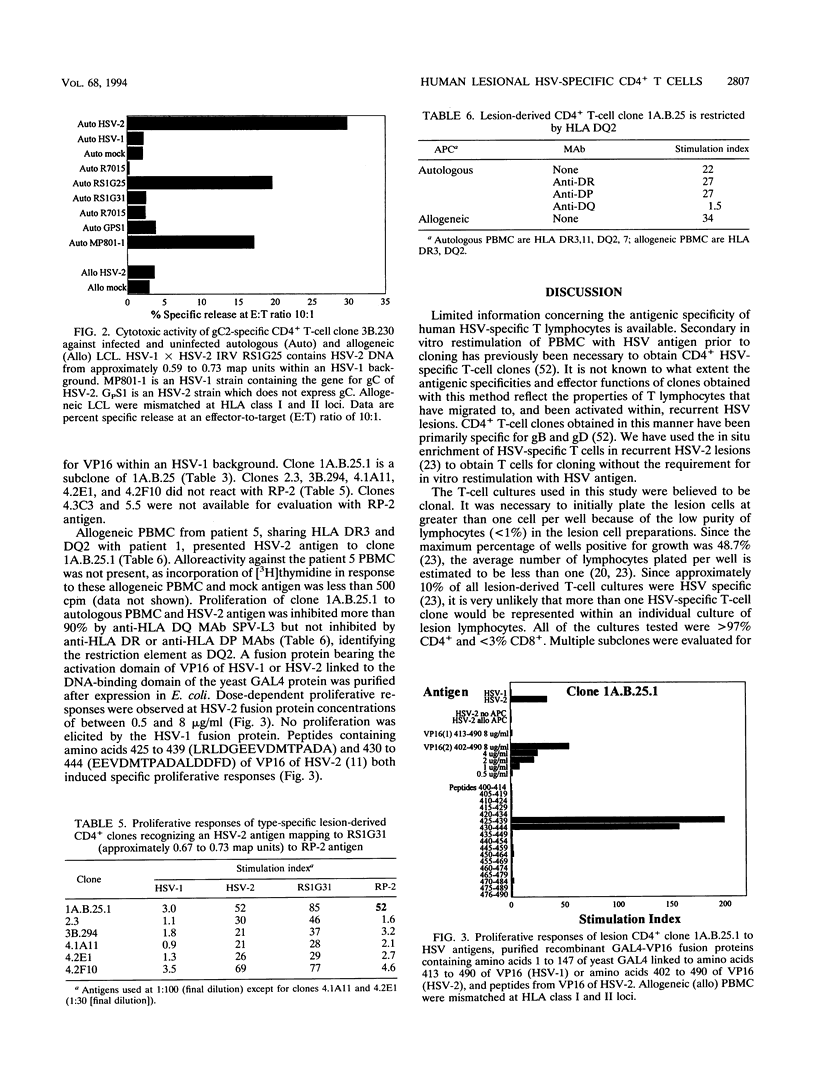
Abstract
Lesions resulting from recurrent genital herpes simplex virus (HSV) infection are characterized by infiltration of CD4+ lymphocytes. We have investigated the antigenic specificity of 47 HSV-specific CD4+ T-cell clones recovered from the HSV-2 buttock and thigh lesions of five patients. Clones with proliferative responses to recombinant truncated glycoprotein B (gB) or gD of HSV-2 or purified natural gC of HSV-2 comprised a minority of the total number of HSV-specific clones isolated from lesions. The gC2- and gD2-specific CD4+ clones had cytotoxic activity. The approximate locations of the HSV-2 genes encoding HSV-2 type-specific CD4+ antigens have been determined by using HSV-1 x HSV-2 intertypic recombinant virus and include the approximate map regions 0.30 to 0.46, 0.59 to 0.67, 0.67 to 0.73, and 0.82 to 1.0 units. The antigenic specificity of an HLA DQ2-restricted, HSV-2 type-specific T-cell clone was mapped to amino acids 425 to 444 of VP16 of HSV-2 by sequential use of an intertypic recombinant virus containing VP16 of HSV-2 in an HSV-1 background, recombinant VP16 fusion proteins, and synthetic peptides. Each of the remaining four patients also yielded at least one type-specific T-cell clone reactive with an HSV-2 epitope mapping to approximately 0.67 to 0.73 map units. The antigenic specificities of lesion-derived CD4+ T-cell clones are quite diverse and include at least 10 epitopes. Human T-cell clones reactive with gC and VP16 are reported here for the first time.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. L., Militoni J., Lee F., Nahmias A., Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988 Apr;26(4):662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley R., Mack K., Critchlow C., Shurtleff M., Corey L. Differential effect of systemic acyclovir treatment of genital HSV-2 infections on antibody responses to individual HSV-2 proteins. J Med Virol. 1988 Mar;24(3):309–319. doi: 10.1002/jmv.1890240308. [DOI] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. Identification of three genes nonessential for growth in cell culture near the right terminus of the unique sequences of long component of herpes simplex virus 1. Virology. 1990 Aug;177(2):684–691. doi: 10.1016/0042-6822(90)90534-x. [DOI] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. The unique sequence of the herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J Virol. 1992 Jan;66(1):562–566. doi: 10.1128/jvi.66.1.562-566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett B. C., Dolan A., Telford E. A., Davison A. J., McGeoch D. J. A novel herpes simplex virus gene (UL49A) encodes a putative membrane protein with counterparts in other herpesviruses. J Gen Virol. 1992 Aug;73(Pt 8):2167–2171. doi: 10.1099/0022-1317-73-8-2167. [DOI] [PubMed] [Google Scholar]
- Batterson W., Roizman B. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J Virol. 1983 May;46(2):371–377. doi: 10.1128/jvi.46.2.371-377.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Guagliardi L. E. The cell biology of antigen processing and presentation. Annu Rev Immunol. 1991;9:707–744. doi: 10.1146/annurev.iy.09.040191.003423. [DOI] [PubMed] [Google Scholar]
- Burke R. L. Development of a herpes simplex virus subunit glycoprotein vaccine for prophylactic and therapeutic use. Rev Infect Dis. 1991 Nov-Dec;13 (Suppl 11):S906–S911. doi: 10.1093/clind/13.supplement_11.s906. [DOI] [PubMed] [Google Scholar]
- Chicz R. M., Urban R. G., Gorga J. C., Vignali D. A., Lane W. S., Strominger J. L. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993 Jul 1;178(1):27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A. J., Knipe D. M., Jones P. C., Roizman B. Molecular genetics of herpes simplex virus. VII. Characterization of a temperature-sensitive mutant produced by in vitro mutagenesis and defective in DNA synthesis and accumulation of gamma polypeptides. J Virol. 1981 Jan;37(1):191–206. doi: 10.1128/jvi.37.1.191-206.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress A., Triezenberg S. J. Nucleotide and deduced amino acid sequences of the gene encoding virion protein 16 of herpes simplex virus type 2. Gene. 1991 Jul 22;103(2):235–238. doi: 10.1016/0378-1119(91)90278-j. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Noble J. R. Role of keratinocytes in human recurrent herpetic lesions. Ability to present herpes simplex virus antigen and act as targets for T lymphocyte cytotoxicity in vitro. J Clin Invest. 1989 Feb;83(2):490–496. doi: 10.1172/JCI113908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. L., Turner R. R., Miller A. C., Para M. F., Merigan T. C. Evolution of recurrent herpes simplex lesions. An immunohistologic study. J Clin Invest. 1985 Jan;75(1):226–233. doi: 10.1172/JCI111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan C. M., Londei M., Corcoran A. E., Grubeck-Loebenstein B., James R. F., Rapoport B., Feldmann M. Autoantigen recognition by thyroid-infiltrating T cells in Graves disease. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7415–7419. doi: 10.1073/pnas.88.16.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebRoy C. Nucleotide sequence of the herpes simplex virus type-2 syn gene that causes cell fusion. Gene. 1990 Apr 16;88(2):275–277. doi: 10.1016/0378-1119(90)90043-q. [DOI] [PubMed] [Google Scholar]
- Elliott G. D., Meredith D. M. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J Gen Virol. 1992 Mar;73(Pt 3):723–726. doi: 10.1099/0022-1317-73-3-723. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Acquisition of specific cytotoxic activity by human T4+ T lymphocytes in culture. Nature. 1984 Mar 22;308(5957):365–367. doi: 10.1038/308365a0. [DOI] [PubMed] [Google Scholar]
- Hendricks R. L., Tumpey T. M., Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1-infected mice. J Immunol. 1992 Nov 1;149(9):3023–3028. [PubMed] [Google Scholar]
- Ho M. Interferon as an agent against herpes simplex virus. J Invest Dermatol. 1990 Dec;95(6 Suppl):158S–160S. doi: 10.1111/1523-1747.ep12875164. [DOI] [PubMed] [Google Scholar]
- Kimura S., Kotani T., McBride O. W., Umeki K., Hirai K., Nakayama T., Ohtaki S. Human thyroid peroxidase: complete cDNA and protein sequence, chromosome mapping, and identification of two alternately spliced mRNAs. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5555–5559. doi: 10.1073/pnas.84.16.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. N., Nash A. A., Sia D. Y., Wildy P. Clonal analysis of T-cell responses to herpes simplex virus: isolation, characterization and antiviral properties of an antigen-specific helper T-cell clone. Immunology. 1984 Dec;53(4):623–633. [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Cunningham C., McIntyre G., Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991 Dec;72(Pt 12):3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Moretta L., Cerottini J. C., Mingari M. C. Direct demonstration of the clonogenic potential of every human peripheral blood T cell. Clonal analysis of HLA-DR expression and cytolytic activity. J Exp Med. 1983 Feb 1;157(2):743–754. doi: 10.1084/jem.157.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Jayasuriya A., Phelan J., Cobbold S. P., Waldmann H., Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987 Mar;68(Pt 3):825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J Virol. 1989 Jan;63(1):196–204. doi: 10.1128/jvi.63.1.196-204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Zezulak K. M., Conley A. J., Weinberger M., Snitzer K., Spear P. G. Use of monoclonal antibodies against two 75,000-molecular-weight glycoproteins specified by herpes simplex virus type 2 in glycoprotein identification and gene mapping. J Virol. 1983 Mar;45(3):1223–1227. doi: 10.1128/jvi.45.3.1223-1227.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves F. C., Spector D., Roizman B. The herpes simplex virus 1 protein kinase encoded by the US3 gene mediates posttranslational modification of the phosphoprotein encoded by the UL34 gene. J Virol. 1991 Nov;65(11):5757–5764. doi: 10.1128/jvi.65.11.5757-5764.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Tognon M. Restriction endonuclease patterns of herpes simplex virus DNA: application to diagnosis and molecular epidemiology. Curr Top Microbiol Immunol. 1983;104:273–286. doi: 10.1007/978-3-642-68949-9_17. [DOI] [PubMed] [Google Scholar]
- Royston I., Omary M. B., Trowbridge I. S. Monoclonal antibodies to a human T-cell antigen and Ia-like antigen in the characterization of lymphoid leukemia. Transplant Proc. 1981 Mar;13(1 Pt 2):761–766. [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Smith L. M., Petty H. R., Parham P., McConnell H. M. Cell surface properties of HLA antigens on Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982 Jan;79(2):608–612. doi: 10.1073/pnas.79.2.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Spruance S. L., Chow F. S. Pathogenesis of herpes simplex labialis. I. Replication of herpes simplex virus in cultures of epidermal cells from subjects with frequent recurrences. J Infect Dis. 1980 Nov;142(5):671–675. doi: 10.1093/infdis/142.5.671. [DOI] [PubMed] [Google Scholar]
- Swain M. A., Peet R. W., Galloway D. A. Characterization of the gene encoding herpes simplex virus type 2 glycoprotein C and comparison with the type 1 counterpart. J Virol. 1985 Feb;53(2):561–569. doi: 10.1128/jvi.53.2.561-569.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M. A., Koelle D., Hartog K., Sekulovich R. E., Corey L., Burke R. L. Human CD8+ herpes simplex virus-specific cytotoxic T-lymphocyte clones recognize diverse virion protein antigens. J Virol. 1992 Mar;66(3):1622–1634. doi: 10.1128/jvi.66.3.1622-1634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torseth J. W., Cohen G. H., Eisenberg R. J., Berman P. W., Lasky L. A., Cerini C. P., Heilman C. J., Kerwar S., Merigan T. C. Native and recombinant herpes simplex virus type 1 envelope proteins induce human immune T-lymphocyte responses. J Virol. 1987 May;61(5):1532–1539. doi: 10.1128/jvi.61.5.1532-1539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson R. J. DNA sequence of the Herpes simplex virus type 2 glycoprotein D gene. Gene. 1983 Dec;26(2-3):307–312. doi: 10.1016/0378-1119(83)90203-2. [DOI] [PubMed] [Google Scholar]
- Weinheimer S. P., Boyd B. A., Durham S. K., Resnick J. L., O'Boyle D. R., 2nd Deletion of the VP16 open reading frame of herpes simplex virus type 1. J Virol. 1992 Jan;66(1):258–269. doi: 10.1128/jvi.66.1.258-269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V. Herpes simplex virus-induced dUTPase: target site for antiviral chemotherapy. Virology. 1988 Sep;166(1):262–264. doi: 10.1016/0042-6822(88)90171-7. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Horiuchi T., Kobayashi Y. Functional heterogeneity among herpes simplex virus-specific human CD4+ T cells. J Immunol. 1991 Feb 15;146(4):1341–1347. [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Kobayashi Y. Helper activity in antigen-specific antibody production mediated by CD4+ human cytotoxic T cell clones directed against herpes simplex virus. J Immunol. 1988 May 15;140(10):3419–3425. [PubMed] [Google Scholar]
- Yasukawa M., Kobayashi Y. Inhibition of herpes simplex virus replication in vitro by human cytotoxic T cell clones and natural killer cell clones. J Gen Virol. 1985 Oct;66(Pt 10):2225–2229. doi: 10.1099/0022-1317-66-10-2225. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Burke R. L., Pachl C., Berman P. W., Lasky L. A. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. IV. Recognition and activation by cloned glycoproteins gB and gD. J Immunol. 1986 Jun 15;136(12):4669–4673. [PubMed] [Google Scholar]
- Zezulak K. M., Spear P. G. Mapping of the structural gene for the herpes simplex virus type 2 counterpart of herpes simplex virus type 1 glycoprotein C and identification of a type 2 mutant which does not express this glycoprotein. J Virol. 1984 Mar;49(3):741–747. doi: 10.1128/jvi.49.3.741-747.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., McKnight J. L. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J Virol. 1993 Mar;67(3):1482–1492. doi: 10.1128/jvi.67.3.1482-1492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaagstra J. C., Leung W. C. The nucleotide sequence of herpes simplex virus type 2 (333) glycoprotein gB2 and analysis of predicted antigenic sites. Can J Microbiol. 1987 Oct;33(10):879–887. doi: 10.1139/m87-154. [DOI] [PubMed] [Google Scholar]


