Abstract
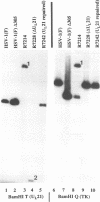
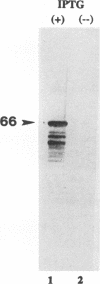
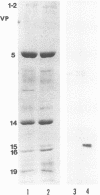
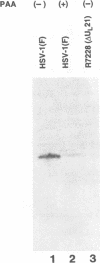
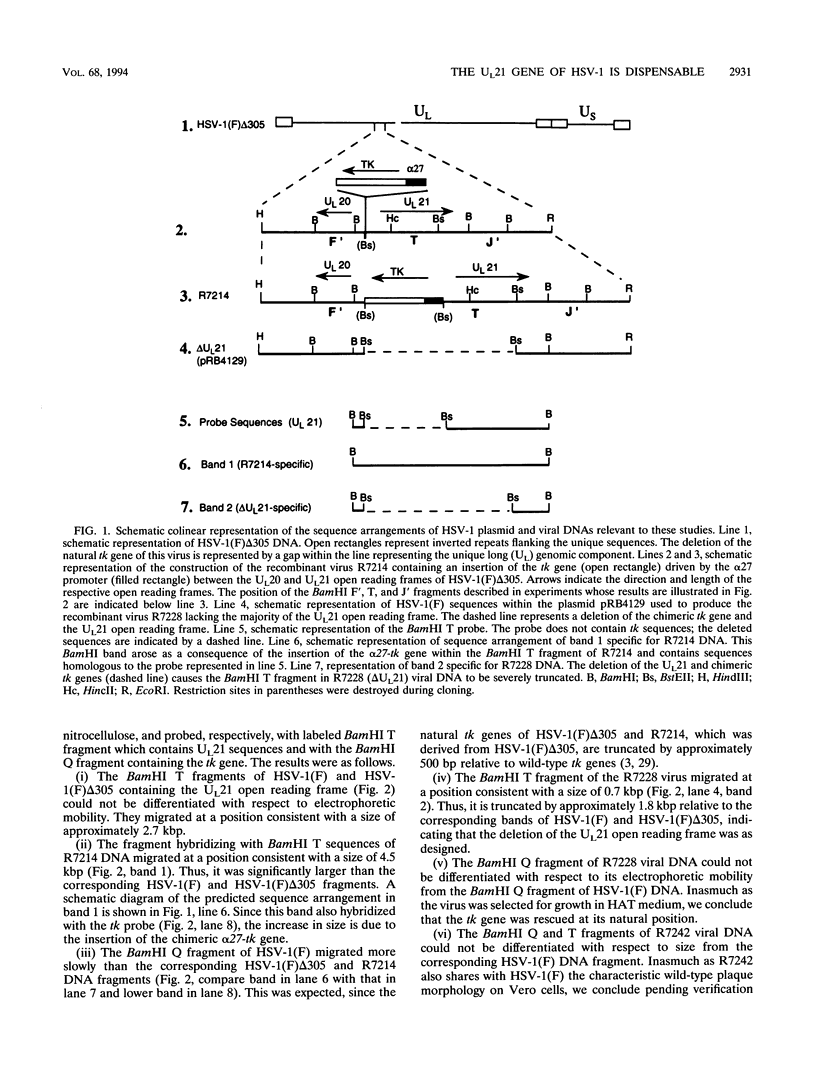
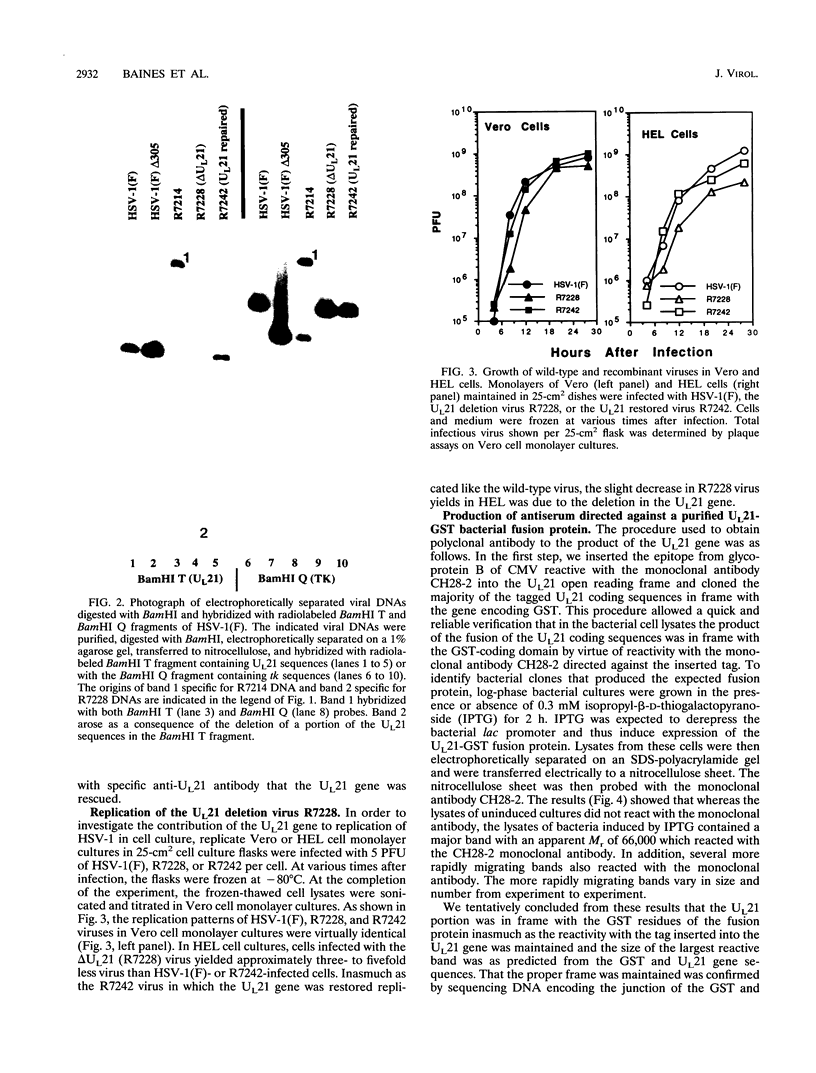
A viral deletion mutant (delta UL21) that lacked the sequences encoding 484 of the predicted first 535 amino acids of the UL21 open reading frame was genetically engineered and studied with respect to its phenotype in cells in culture. We report the following. (i) The replication of delta UL21 was identical to that of the parent herpes simplex virus 1 (HSV-1) strain F in Vero cells, but the yields were three- to fivefold lower than those of the parent virus in human embryonic lung cells. (ii) To characterize the UL21 protein, we immunized rabbits against a purified bacterial fusion protein consisting of glutathione S-transferase fused to the majority of the coding domain of the UL21 gene. Rabbit antiserum directed against the fusion protein recognized a broad band with an apparent M(r) of 62,000 to 64,000 in lysates of cells infected with HSV-1 strain F and in virions purified from the infected cell cytoplasm. This band was absent from lysates of mock-infected cells or cells infected with the delta UL21 virus. The band was significantly reduced in intensity in lysates of cells infected in the presence of phosphonoacetic acid, indicating that it is expressed as a late (gamma 1) gene. (iii) Immunofluorescence studies localized the UL21 antigen primarily in brightly staining granules in the cytoplasms of infected cells. Taken together, the data indicate that the UL21 protein is a virion component dispensable for all aspects of replication of HSV-1 in the cells tested. The electrophoretic mobility of the UL21 protein suggests that it is extensively modified posttranslationally.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Chou J., Sarmiento M., Lerner R. A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986 Jun;58(3):843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel viral glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993 Mar;67(3):1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J Virol. 1992 Aug;66(8):5168–5174. doi: 10.1128/jvi.66.8.5168-5174.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991 Feb;65(2):938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines J. D., Ward P. L., Campadelli-Fiume G., Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991 Dec;65(12):6414–6424. doi: 10.1128/jvi.65.12.6414-6424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. Identification of three genes nonessential for growth in cell culture near the right terminus of the unique sequences of long component of herpes simplex virus 1. Virology. 1990 Aug;177(2):684–691. doi: 10.1016/0042-6822(90)90534-x. [DOI] [PubMed] [Google Scholar]
- Barker D. E., Roizman B. The unique sequence of the herpes simplex virus 1 L component contains an additional translated open reading frame designated UL49.5. J Virol. 1992 Jan;66(1):562–566. doi: 10.1128/jvi.66.1.562-566.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basgoz N., Qadri I., Navarro D., Sears A., Lennette E., Youngblom J., Pereira L. The amino terminus of human cytomegalovirus glycoprotein B contains epitopes that vary among strains. J Gen Virol. 1992 Apr;73(Pt 4):983–988. doi: 10.1099/0022-1317-73-4-983. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Cohen G., Eisenberg R., Long D., Wagner E. Direct demonstration that the abundant 6-kilobase herpes simplex virus type 1 mRNA mapping between 0.23 and 0.27 map units encodes the major capsid protein VP5. J Virol. 1984 Jan;49(1):287–292. doi: 10.1128/jvi.49.1.287-292.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M. D., Rixon F. J., Davison A. J. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex virus type 1. J Gen Virol. 1992 Oct;73(Pt 10):2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Everett R. D. Inactivation of the shutoff gene (UL41) of herpes simplex virus types 1 and 2. J Gen Virol. 1990 Dec;71(Pt 12):2961–2967. doi: 10.1099/0022-1317-71-12-2961. [DOI] [PubMed] [Google Scholar]
- Fisher F. B., Preston V. G. Isolation and characterisation of herpes simplex virus type 1 mutants which fail to induce dUTPase activity. Virology. 1986 Jan 15;148(1):190–197. doi: 10.1016/0042-6822(86)90414-9. [DOI] [PubMed] [Google Scholar]
- Georgopoulou U., Michaelidou A., Roizman B., Mavromara-Nazos P. Identification of a new transcriptional unit that yields a gene product within the unique sequences of the short component of the herpes simplex virus 1 genome. J Virol. 1993 Jul;67(7):3961–3968. doi: 10.1128/jvi.67.7.3961-3968.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The promoter, transcriptional unit, and coding sequence of herpes simplex virus 1 family 35 proteins are contained within and in frame with the UL26 open reading frame. J Virol. 1991 Jan;65(1):206–212. doi: 10.1128/jvi.65.1.206-212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker R., Roizman B. Generation of an inverting herpes simplex virus 1 mutant lacking the L-S junction a sequences, an origin of DNA synthesis, and several genes including those specifying glycoprotein E and the alpha 47 gene. J Virol. 1986 May;58(2):583–591. doi: 10.1128/jvi.58.2.583-591.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C. A., Dolan A., Jamieson F. E., McGeoch D. J. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J Gen Virol. 1992 Mar;73(Pt 3):539–547. doi: 10.1099/0022-1317-73-3-539. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Brauer D. H. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986 Feb 25;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dolan A., Donald S., Rixon F. J. Sequence determination and genetic content of the short unique region in the genome of herpes simplex virus type 1. J Mol Biol. 1985 Jan 5;181(1):1–13. doi: 10.1016/0022-2836(85)90320-1. [DOI] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992 May;66(5):2653–2663. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Roizman B. In vivo behavior of genetically engineered herpes simplex viruses R7017 and R7020: construction and evaluation in rodents. J Infect Dis. 1988 Sep;158(3):602–614. doi: 10.1093/infdis/158.3.602. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Post L. E., Roizman B. A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981 Jul;25(1):227–232. doi: 10.1016/0092-8674(81)90247-6. [DOI] [PubMed] [Google Scholar]
- Purves F. C., Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein alpha 22. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Davison M. D., Davison A. J. Identification of the genes encoding two capsid proteins of herpes simplex virus type 1 by direct amino acid sequencing. J Gen Virol. 1990 May;71(Pt 5):1211–1214. doi: 10.1099/0022-1317-71-5-1211. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visalli R. J., Brandt C. R. The HSV-1 UL45 gene product is not required for growth in Vero cells. Virology. 1991 Nov;185(1):419–423. doi: 10.1016/0042-6822(91)90790-i. [DOI] [PubMed] [Google Scholar]
- Wadsworth S., Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975 Jun;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Seghatoleslami M. R., Shao L., Rowse D., Carmichael E. P. The herpes simplex virus type 1 alkaline nuclease is not essential for viral DNA synthesis: isolation and characterization of a lacZ insertion mutant. J Gen Virol. 1990 Dec;71(Pt 12):2941–2952. doi: 10.1099/0022-1317-71-12-2941. [DOI] [PubMed] [Google Scholar]
- Yei S. P., Chowdhury S. I., Bhat B. M., Conley A. J., Wold W. S., Batterson W. Identification and characterization of the herpes simplex virus type 2 gene encoding the essential capsid protein ICP32/VP19c. J Virol. 1990 Mar;64(3):1124–1134. doi: 10.1128/jvi.64.3.1124-1134.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Sirko D. A., McKnight J. L. Role of herpes simplex virus type 1 UL46 and UL47 in alpha TIF-mediated transcriptional induction: characterization of three viral deletion mutants. J Virol. 1991 Feb;65(2):829–841. doi: 10.1128/jvi.65.2.829-841.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wind N., Wagenaar F., Pol J., Kimman T., Berns A. The pseudorabies virus homology of the herpes simplex virus UL21 gene product is a capsid protein which is involved in capsid maturation. J Virol. 1992 Dec;66(12):7096–7103. doi: 10.1128/jvi.66.12.7096-7103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]