Abstract
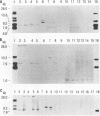
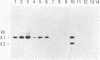
Several groups of wild mice (Mus musculus) were captured from eight different locations in Asia and bred for several generations in a facility free of any laboratory strains of mice carrying mouse mammary tumor virus (MMTV). The distribution of endogenous MMTV proviral sequences in the liver tissues of these mice was investigated by using Southern blot hybridizations. Four categories of mice were identified. Mice originating from Bogor, Indonesia (Cas-Bgr); He-mei, Taiwan (Cas-Hmi/1); and Malaysia (Cas-Mal) were found to carry an endogenous MMTV provirus consisting of the env, gag-pol, and long terminal repeat sequences. Mice captured from Kojuri, Republic of Korea (Sub-Kjr); Nagoya, Japan (Mol-nag); and three Chinese provinces, Shanghai (Sub-Shh), Beijing (Sub-Bjn), and Jiayuguang (Sub-Jyg/1), appeared to carry defective proviruses. Some mice originating from He-mei (Cas-Hmi/2) and Jiayuguang (Sub-Jyg/2) were found to be completely free of endogenous MMTV. Interestingly, however, the Sub-Jyg/2 mice, after several generations of inbreeding, were found, unlike all of the other subspecies that we examined in the present study, to develop mammary tumors at a high incidence (80 to 90%) with a short period of latency. Electron microscopic examination of the mammary glands and mammary tumors of these mice revealed the presence of numerous intracytoplasmic A, immature, budding, and mature B particles. Furthermore, the mammary tumors were found to contain MMTV proviral sequences. It seems, therefore, that Sub-Jyg/2 mice carry an exogenous MMTV which contributes to their developing mammary tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERVONT H. B. Biological studies on the mammary tumor inciter in mice. Ann N Y Acad Sci. 1952 Jul 10;54(6):1004–1011. doi: 10.1111/j.1749-6632.1952.tb39975.x. [DOI] [PubMed] [Google Scholar]
- ANDERVONT H. B., DUNN T. B. Occurrence of tumors in wild house mice. J Natl Cancer Inst. 1962 May;28:1153–1163. [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Sherr C. J., Schidlovsky G., Todaro G. J. A new class of genetically transmitted retravirus isolated from Mus cervicolor. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3579–3583. doi: 10.1073/pnas.73.10.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Drohan W., Gallahan D., D'Hoostelaere L., Potter M. Novel class of mouse mammary tumor virus-related DNA sequences found in all species of Mus, including mice lacking the virus proviral genome. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4113–4117. doi: 10.1073/pnas.79.13.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C. Molecular aspects of mouse mammary tumor virus biology. Int Rev Cytol. 1987;108:119–147. doi: 10.1016/s0074-7696(08)61437-0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Escot C., Hogg E., Callahan R. Mammary tumorigenesis in feral Mus cervicolor popaeus. J Virol. 1986 May;58(2):619–625. doi: 10.1128/jvi.58.2.619-625.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkind P. R., Sarkar N. H. Integration of new endogenous mouse mammary tumor virus proviral DNA at common sites in the DNA of mammary tumors of C3Hf mice and hypomethylation of the endogenous mouse mammary tumor virus proviral DNA in C3Hf mammary tumors and spleens. J Virol. 1983 Jan;45(1):114–123. doi: 10.1128/jvi.45.1.114-123.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., Morimoto J., Tsubura Y., Iwai Y., Okumoto M., Takamori Y., Tsubura A., Hilgers J. Tissue and organ distribution of mammary tumor virus antigens in low and high mammary cancer strain mice. Eur J Cancer Clin Oncol. 1983 Jul;19(7):1011–1019. doi: 10.1016/0277-5379(83)90071-8. [DOI] [PubMed] [Google Scholar]
- Imai S., Tsubura Y., Hilgers J., Michalides R. A new locus (Mtv-4) for endogenous mammary tumor virus expression and early mammary tumor development in the SHN mouse strain. J Natl Cancer Inst. 1983 Sep;71(3):517–521. [PubMed] [Google Scholar]
- Kozak C., Peters G., Pauley R., Morris V., Michalides R., Dudley J., Green M., Davisson M., Prakash O., Vaidya A. A standardized nomenclature for endogenous mouse mammary tumor viruses. J Virol. 1987 May;61(5):1651–1654. doi: 10.1128/jvi.61.5.1651-1654.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti A., Robbins J., Campbell G., Buttitta F., Squartini F., Bistocchi M., Callahan R. Host genetic background effect on the frequency of mouse mammary tumor virus-induced rearrangements of the int-1 and int-2 loci in mouse mammary tumors. J Virol. 1991 Aug;65(8):4550–4554. doi: 10.1128/jvi.65.8.4550-4554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey R. J., Arthur L. O., Nowinski R. C., Schochetman G. Monoclonal antibodies identify individual determinants on mouse mammary tumor virus glycoprotein gp52 with group, class, or type specificity. J Virol. 1980 Jun;34(3):635–643. doi: 10.1128/jvi.34.3.635-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A., Moon R., Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991 Jan 25;64(2):231–231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Nusse R. The int genes in mammary tumorigenesis and in normal development. Trends Genet. 1988 Oct;4(10):291–295. doi: 10.1016/0168-9525(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Peters G. Oncogenes at viral integration sites. Cell Growth Differ. 1990 Oct;1(10):503–510. [PubMed] [Google Scholar]
- Sarkar N. H., Moore D. H. Electron microscopy in mammary cancer research. J Natl Cancer Inst. 1972 Apr;48(4):1051–1058. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tsubura A., Inaba M., Imai S., Murakami A., Oyaizu N., Yasumizu R., Ohnishi Y., Tanaka H., Morii S., Ikehara S. Intervention of T-cells in transportation of mouse mammary tumor virus (milk factor) to mammary gland cells in vivo. Cancer Res. 1988 Nov 15;48(22):6555–6559. [PubMed] [Google Scholar]
- Yonekawa H., Gotoh O., Tagashira Y., Matsushima Y., Shi L. I., Cho W. S., Miyashita N., Moriwaki K. A hybrid origin of Japanese mice "Mus musculus molossinus". Curr Top Microbiol Immunol. 1986;127:62–67. [PubMed] [Google Scholar]